Abstract
Among 2D materials (Xenes) which are at the forefront of research activities, borophene, is an exciting new entry due to its uniquely varied optical, electronic, and chemical properties in many polymorphic forms with widely varying band gaps including the lightest 2D metallic phase. In this paper, we used a simple selective chemical etching to prepare borophene with a strong near IR light-induced photothermal effect. The photothermal efficiency is similar to plasmonic Au nanoparticles, with the added benefit of borophene being degradable due to electron deficiency of boron. We introduce this selective chemical etching process to obtain ultrathin and large borophene nanosheets (thickness of ~4 nm and lateral size up to ~600 nm) from the precursor of AlB2. We also report first-time observation of a selective Acid etching behavior showing HCl etching of Al to form a residual B lattice, while HF selectively etches B to yield an Al lattice. We demonstrate that through surface modification with polydopamine (PDA), a biocompatible smart delivery nanoplatform of B@PDA can respond to a tumor environment, exhibiting an enhanced cellular uptake efficiency. We demonstrate that borophene can be more suitable for safe photothermal theranostic of thick tumor using deep penetrating near IR light compared to gold nanoparticles which are not degradable, thus posing long-term toxicity concerns. With about 40 kinds of borides, we hope that our work will open door to more discoveries of this top-down selective etching approach for generating borophene structures with rich unexplored thermal, electronic, and optical properties for many other technological applications.
Similar content being viewed by others
Introduction
Light has made profound impacts on clinical practice for diagnosis and treatment. The modern use of light in medicine went through a rapid improvement in the understanding of fundamental light–matter interactions since the 19th century. When absorbed by a biological tissue or an added exogenous probe, the resulting emission shows the microstructure and ingredient of the tissue, serving for optical imaging and diagnostics. On the other hand, the photoirradiation of the tissue through introduced photosensitizer can also be used in therapy via photothermal effect, photochemical reactions (photodynamic effect), or another biological process (optogenetic).
Recent advances in biomedical optics have enabled integrated photonics technologies with nanotechnology and biomaterials. The two-dimensional (2D) nanomaterials, inspired by the great success of graphene1, have undergone tremendous expansion in recent years to include MoS22,3,4,5, MXenes6,7,8,9, black phosphorus (BP)10,11,12,13,14,15,16,17,18,19,20,21, bismuthene22,23,24, antimonene25,26,27,28, tellurene29,30,31,32,33,34,35, selenene36,37, BP-analog materials38,39,40,41, 2D metal42,43, MOF44,45, etc. Recently, borophene, as the neighbor of graphene, has aroused great attention because it is the lightest 2D material up to now and is revealing many unique properties different from graphene46,47. With polymorphism and anisotropic structure48,49,50, borophene contributes to numerous unique characteristics such as mechanical flexibility51,52, metallicity53, optical transparency54, etc. Metallicity is the most unique attribute of borophene in comparison with semimetals or semiconductor, and thus it is promising for optically transparent electrode54. On the contrary, the semiconducting property can also be found in the γ-B28 phase monolayer and a strong photoluminescence signal is observed at ~626 nm corresponding to its direct band gap of 2.25 eV55. Moreover, the combined result of low mass and small bending stiffness is high phonon velocity, leading to efficient thermal transport56.
As is well-known, owing to a covalent bonding framework in bulk boron, borophene fabrication remains a great challenge. Theoretically, a triangular lattice with periodic holes, grown on metal substrates49,57, is predicted to be more stable58,59. This striped-phase borophene was first demonstrated experimentally through molecular beam epitaxy (MBE) growth on Ag(111) by Mannix et al.53 under ultrahigh-vacuum conditions. Moreover, the chemical vapor deposition (CVD) was used to grow atomically thin borophene on Cu foils55. In addition to these bottom-up growth, a top-down strategy was employed by Ji et al.60 to prepare borophene through synergetic thermal oxidation and liquid-phase exfoliation. Sonochemical technique was also used to exfoliate bulk boron by Ranjan et al.61. The rare bottom-up fabrication investigations illustrate the difficulty in fabrication of borophene from non-layer bulk B and thus limit any scope of wide application.
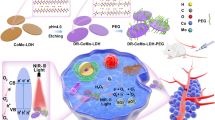
Herein, we demonstrate the light-induced tumor theranostics of the newly-fabricated borophene, as shown in Fig. 1. The borophene was produced by employing acid selective etching of the AlB2 precursor. A surprising finding is that while HCl etching produces borophene by dissolving Al, etching by HF yields Al sheets by dissolving B. Our unique method can overcome the hurdle in direct exfoliation from the covalently-bonding bulk boron. Similar to the exfoliation of MXene62,63,64, ultrathin and large borophene nanosheets can be obtained with a high yield. The relatively mild etching environment maintains the integrity of the borophene structure. This as-prepared borophene has significant absorption at 800 nm in the near IR region to produce the significant photothermal effect which can be used for photoacoustic imaging and photothermal cancer therapy. Using surface modification with polydopamine (PDA), we developed a biocompatible smart delivery nanoplatform of B@PDA which exhibits low toxicity, enhanced cellular uptake, strong photoacoustic signal at 800 nm, and photothermal therapy.
Results
Fabrications
For the etching of borophene, suitable etching agent needs to be chosen. The etching efficacy of HCl and HF were compared. It was surprising to find that the boron ingredient can be obtained and aluminum can be etched out using the HCl etching process, while it is the opposite situation for the HF etching process (Fig. 2). As shown in Fig. 2a, the samples for HCl etching and HF etching show different X-ray diffraction (XRD) peaks from the precursor of AlB2. For the HCl etched sample, the most intense XRD peaks of AlB2 vanish and a peak at a small 2θ of 6o appears, showing the ultrathin dimension of the B product exfoliated from bulk AlB2. The sample after HF etching shows peaks similar to the XRD pattern to AlF365 and Al2O366, with a strong XRD peak at 2θ of ~8o indicative of ultrathin nanosheets. The chemical composition for both etching products was characterized by X-ray photoelectron spectroscopy (XPS) (Fig. 2b)67,68. After HCl etching, it is observed that the main component is the boron element68, while the HF etched product revealed Al, O and F elements, illustrated by the observed typical XPS patterns (Figs. 2b and S1)69. The energy-dispersive X-ray spectroscopy (EDS) mapping in the scanning transmission electron microscopy (STEM) mode shows the B, O without Al elements after HCl etching (Fig. 2c) and the Al, F, and O elements without B after HF etching (Fig. 2d). The quantitative analysis of the elemental composition is further performed by EDS analysis (Fig. S2). It is clearly observed that the B element accounts for 95.88% of weight after HCl etching, while HF etching can completely remove the B element, leaving Al, O and F elements. These systematic characterizations collectively prove that acid selective etching is an efficient strategy for achieving a boron product from its precursor of AlB2 and HCl is a suitable etching agent.
Characterizations
After confirming a successful chemical etching process, morphological characterizations of the etched products are further conducted (Fig. 3). Figure 3a shows the SEM image of bulk AlB2. After HCl etching, a book page-like structure can be observed, showing successful extraction of the Al layer from AlB2 (Fig. 3b). The insets in Fig. 3a, b show the stark color contrast before and after etching. Further, with the sonication process, large and ultrathin borophene nanosheets with several hundreds of nm can be exfoliated (Fig. 3c, d). In particular, the nanosheets appear to be hexagonal, which may be due to the honeycomb structure in bulk AlB270. The thickness of the borophene nanosheets is measured to be ~4 nm (Fig. 3e, f), exhibiting the high aspect ratio of lateral size and thickness.
a Scanning electron microscope (SEM) image of bulk AlB2. Inset shows the black color of bulk AlB2 before etching. b Book page-like morphology after HCl etching. Inset shows the red color of etching products from AlB2. c, d Transmission electron microscope (TEM) and SEM image of the borophene nanosheets. e, f The ultrathin thickness of borophene nanosheets characterized by atomic force microscope (AFM). g Crystal lattice characterized by the high-resolution TEM. h Selected-area electron diffraction (SAED) of borophene. The blue rectangle and green triangle correspond to the β12 structure and χ3 structure, respectively
Through the high-resolution TEM image (left part, Fig. 3g), the interatomic distance can be observed to be 0.54 nm, which agrees with the β12 model of borophene67. For the right part, the distance of 0.26 nm indicates the occurrence of also the χ3 phase. The diffraction pattern (Fig. 3h) further proves the coexistence of both the β12 and χ3 phases. Such an intermediate phase may be formed because of the absence of a supporting substrate, which is similar to the case of sonochemical exfoliated borophene61. Therefore, the final borophene phase depends only on the energetics of the lattice. The β12 phase possesses an out-of-plane structure with minimum energy. This metastable structure of the mixed-phase might have higher energy than that of β12, maintained by the special etching solution environment.
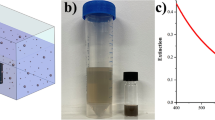
Furthermore, the photothermal performance of the as-prepared borophene is characterized13,43,71,72. For the naked borophene, the dispersions for different concentrations are shown in Fig. 4a and the corresponding absorbance is shown in Fig. 4b. The extinction coefficient (k) is determined to be 6.0 Lg−1cm−1 (Fig. 4c), comparable to that of Ge nanosheets (5.962 Lg−1cm−1)73 and higher than those of gold nanorods (3.9 Lg−1cm−1)74 and antimonene (3.6 Lg−1cm−1)75, as shown in Table S1. To further enhance the absorption and biocompatibility, polydopamine (PDA) is chosen to decorate the borophene76,77,78. For PDA decorated borophene, the extinction coefficient can be enhanced to be 6.5 Lg−1cm−1 at 808 nm (Fig. 4d, e). In photothermal study, the temperature of B@PDA increases much faster than in pure PDA as laser irradiation time increases (Figs. 4f and S6). A gradually decaying photothermal performance of B@PDA for six cycles can be observed (Fig. 4g) indicating gradual degradation of the nanostructure. The absorbance decrease after laser irradiation also proved the degradability of B@PDA (Fig. 4h). The photothermal efficiency is calculated to be 32% (Fig. 4i), which is higher than MoS279 and Ti3C280 nanosheets (Table S2). The photothermal performance of the as-prepared materials at different pH values were also investigated (Fig. S3). The results show that acidic conditions slightly affect the photothermal properties of B@PDA, which may be related to accelerated degradation. Notably, the B@PDA possess photothermal performance upon NIR II laser (1064 nm) irradiation, but weakly compared to 808 nm irradiation (Figs. S4 and S5).
a–c Photograph, absorbance, and extinction coefficient of naked borophene. d, e Absorbance and extinction coefficient of B@PDA. f Photothermal temperature increases at different concentrations of B@PDA. g Periodic photothermal cycles. h Stability is shown by the absorbance before and after irradiation. i The calculation for photothermal conversion efficiency
In vitro anticancer efficacy assay of B@PDA
Good biocompatibility of B@PDA is the first requisite for its biomedical application. Therefore, in vitro cytotoxicity of B@PDA was tested in several different tumor cells, including HCT-116, HeLa, A549 and MCF7. The CCK-8 assay was used to measure the cells’ viability. These cells were incubated with B@BPA at different concentrations of 25–500 ppm for 48 h and no obvious cytotoxicity was observed for the tested cells even at the high concentration of 500 ppm (Fig. 5a). This result proves the low cytotoxicity of B@PDA and its potential for biomedicine in the first step, which was also verified on noncancerous cells (Figs. S10 and S11). After this confirmation, the photothermal killing effect of the B@PDA was further checked. The cells were firstly cultured with B@PDA for 4 h, and then were irradiated using an 808 nm laser (1 W cm−2, 10 min). The CCK-8 assays were used to measure the cell viability after another 24 h’s incubation. Figure 5b demonstrates a dose-dependent photothermal killing effect in HCT-116 cells. Figure 5c–e show similar results on other cells. In contrast, pure PDA exhibited negligible cell photothermal killing effect, probably because most of the PDA was lost during washing (Fig. S9). A laser scanning confocal microscopy (LSCM) assay in HCT-116 cancer cells provides similar results through staining cells after laser irradiation. The red fluorescence represents dead cells from propidium iodide (PI) and the green fluorescence represents live cells from Calcein-AM (Fig. 5f), which shows the obvious photothermal killing effect of the B@PDA.
a Relative viability of HCT-116, HeLa, A549, and MCF7 cells after exposure in B@PDA-based medium (25–500 ppm) for 48 h. b Relative viability of HCT-116 cell incubated with B@PDA (1.5, 3, 6, 12, 24, 48, and 96 ppm) for 4 h and after photothermal treatment (1 W cm−2, 808 nm, 10 min,). c–e were treated with the same condition as (b) for HeLa, MCF7, and A549, respectively. f Corresponding laser scanning confocal microscopy (scale bars, 50 µm for all panels) of HCT-116 cells stained with PI (dead cells, red) and calcein AM (live cells, green)
Endocytosis
PDA with pH-sensitive property is promising for weakly acidic tumor microenvironment delivery76,77,78. To show the photothermal killing mechanism of B@PDA, the intracellular tracking of B@PDA was made through a coating of Cy5. The uptake ability of Cy5-B@PDA by HCT-116 cells in different pH environments (pH 7.4 and 6.5) was observed by LSCM. After treatment with Cy5-B@PDA for 15 min, the intracellular fluorescent intensities of Cy5-B@PDA at pH 6.5 and 7.4 environments were similar. Interestingly, the fluorescence of Cy5-B@PDA at pH 6.5 (Fig. 6a(a)) was stronger than that at pH 7.4 after 3 h (Fig. 6a(b)). This result demonstrates that PDA can facilitate cellular penetration of B@PDA in the acid tumor environment.
When HCT-116 was incubated with Cy5-B@PDA for 30 min, red Cy5 fluorescence was observed in the mitochondria (Fig. 6b(a)). After 3 h’s incubation of Cy5-B@PDA with HCT-116 cells, the red Cy5 fluorescence increased significantly (Fig. 6b(b)). The above results demonstrated that Cy5-B@PDA could target mitochondria, and further facilitate the following reactive oxygen species (ROS)-based therapy.
The ROS are highly reactive species produced from O2 metabolism81,82. The toxicity mechanism of some inorganic nanomaterials is related to ROS83. The level of ROS induced by B@PDA treatment of HCT-116 cells without laser irradiation was higher than the control (Fig. 7a, b). In the NIR laser irradiated cells, the ROS value increased considerably compared with the control (Fig. 7c). In the B@PDA + NIR group, the induced ROS quantity is the maximum (Fig. 7d). Therefore, the B@PDA-based tumor cell killing can be attributed to the production of ROS.
In vivo imaging
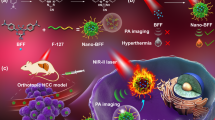
It was revealed that nanomaterials induce endothelial leakiness (NanoEL) and exhibit enhanced permeability and retention (EPR) effect, leading to enhanced tumor-targeting ability84,85,86. The in vivo theranostic efficacy of the B@PDA-based platform was further explored. Recently, photoacoustic (PA) imaging has shown its potential in photothermal-based acoustic imaging technique in deep tumor87,88,89. Since the as-prepared B@PDA exhibits excellent photothermal performance, the B@PDA was further tested for PA imaging. Figure 8a shows a dose-dependent PA signal. A linear relation of the PA signal with the concentration of B@PDA was observed in Fig. 8b. In contrast, the photoacoustic signal of pure PDA is significantly weaker than that of B@PDA (Figs. S7 and S8). For the in vivo test, mice bearing HCT-116 tumor were injected with B@PDA systemically through caudal vein injection. After injection, the PA signal was measured at time points of 0, 6, and 24 h. A strong PA signal was observed 24 h post-injection of B@PDA at the tumor site (Fig. 8c). Moreover, a quantitative calculation of the PA signal further proved the strongest signal intensity at 24 h (Fig. 8d). These characterizations showed that B@PDA can be employed as a good PA contrast agent for guiding tumor therapy.
a In vitro PA images of B@PDA vs. concentration (0, 0.125, 0.25, 0.5, 1, and 2 µg µl−1). b PA images of B@PDA vs. concentration (0, 0.125, 0.25, 0.5, 1, and 2 µg µl−1). c PA aqasximages in tumor site at time points of 1, 6, and 24 h post-injection. d Quantitative calculation of PA signal in PA images (c). e In vivo fluorescence images in tumor site of Cy5.5-labeled B@PDA during 24 h. f Quantitative calculation of Cy5.5-labeled B@PDA locating in the tumor site. g Fluorescence images of major organs at 24 h post-injection. h Quantitative biodistribution of B@PDA in BALB/c mice
Furthermore, fluorescence imaging of animals was also conducted. The fluorescence signal in tumor sites at time points of 0, 12, and 24 h was measured using an in vivo imaging system. The very strong fluorescent signal appears post 24 h caudal vein injection of Cy5.5-labeled B@PDA at the tumor site (Fig. 8e, f). Then, the mice were killed and the organs were collected for fluorescence imaging. The tumor tissue shows the strongest fluorescent signal (Fig. 8g). Quantitative biodistribution of Cy5.5-labeled B@PDA in BALB/c mice was measured (Fig. 8h), which further confirmed the tumor-targeting ability of B@PDA. Therefore, a multi-modal imaging platform based on B@PDA can be achieved.
Toxicity
Before in vivo treatments, the toxicity of B@PDA was evaluated through in vivo test. The mice were injected with B@PDA and PBS intravenously (i.v.), respectively. For immune analysis, serum samples were gathered at 2 h and 24 h after injection. The IL-6, IL-12+P40, TNF-α, and IFN-γ levels in the B@PDA group were almost the same as those of the PBS group (Fig. 9a–d), demonstrating that B@PDA cannot cause any evident cytokine response. In the histology and hematology assay, the healthy mice were i.v. injected with B@PDA and PBS. The blood was gathered at 1, 7, and 14 days post-injection. The serum biochemical index was further measured, including urea nitrogen (BUN), alkaline phosphatase (ALP), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) (Fig. 9e–h). For blood routine analysis, red blood cells (RBC), hemoglobin (HGB), white blood cells (WBC), mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), mean corpuscular hemoglobin (MCH), platelet (PLT), neutrophil (NEU), hematocrit (HCT), creatinine (Cr), mean platelet volume (MPV), and lymphocyte (LYM) counts were detected (Fig. 9i–t). No significant statistical difference in all the parameters was observed between B@PDA and PBS-treated groups. These results show that B@PDA does not induce any obvious inflammation in BALB/c mice. Moreover, the H&E staining shows no distinct tissue damage sign in major organs (Fig. 9u).
In vivo photothermal therapy
Based on the excellent in vivo biocompatibility of the B@PDA platform, the in vivo antitumor study was carried out90. The mice with HCT-116 tumor were separated into four groups with different treatments: Group 1: PBS (control), Group2: B@PDA, Group 3: NIR (808 nm) and Group 4: B@PDA + NIR (808 nm). The intravenously injected dose of B@PDA was 5 mg kg−1 in related groups. Groups 3 and 4 were irradiated with an 808 nm laser (1 W cm−2, 10 min). After these treatments, the width and length of tumor were measured every 2 days and then calculated (Fig. 10a). Digital photos and weighs of representative tumors were also taken (Fig. 10b, c). Compared with the Control group, all Groups 2–4 exhibit tumor growth inhibition. Notably, Group 4 showed a better inhibition effect of tumor growth, demonstrating the enhanced therapeutic effect. Moreover, it is observed that there are no obvious side effects in the treatment process through weight measurement (Fig. 10d).
a Digital images of representative HCT-116 tumors after treatment. b Tumor weight comparison after treatment for four groups. c Tumor volume change during treatment (n = 4). d Mouse weight change during treatment. Error bar and mean value are defined as s.d. and mean, respectively. P-value was calculated by two-tailed Student’s t test (***P < 0.005, *P < 0.05)
Discussion
For the first time, we found that a top-down etching method can be used to fabricate borophene from a boride precursor and demonstrated its light-induced tumor therapy and imaging function. Ultrathin and large borophene nanosheets are successfully exfoliated. Through decoration of borophene with PDA, an effective cellular targeting system in the tumor environment is obtained. Besides novel fabrication, dual-modal photoacoustic and fluorescence imaging can be achieved to prove the tumor aggregation ability and facilitation of the phototherapy.
The novel fabrication technique of borophene as introduced here can be expanded to many other borides (MB2, M = Ti, Cr, V, Mn, Nb, Zr, Hf, Mo, etc.)91. Many unexplored borophene structures can be obtained through this fabrication technique as candidates for further light-induced tumor therapy and diagnosis. Other light-induced effects can also be explored, like photochemical, photomechanical effects or photobiological effects. Besides the tumor related theranostics, light interacting with these novel materials can provide more possibilities, such as treatment of cutaneous disorders, surgeries in ophthalmology, and interventional therapy in internal organs through fiber-optic delivery.
Materials and methods
Fabrications
The bulk AlB2 powder was bought from Sigma-Aldrich company. In all, 1 g of bulk AlB2 was added into HCl (30 mg/ml), respectively. Then the dispersion was stirred at 50 °C for 12 h. After reaction, the mixture was involved in an ultrasonic treatment. Finally, the borophene can be obtained through a centrifugation range of 3000 to 10000 rpm. The resulting sediment was then washed in deionized water. For HF etching, the method is the same.
1 mg of prepared borophene was dispersed with 1 mL ethanol. Dopamine hydrochloride aqueous solution (10 μL, 150 mg mL−1) and NaOH aqueous solution (50 μL, 10 mg mL−1) were mixed. Then, the mixture was stirred in dark for 2.5 h. Finally, the dispersion was centrifuged at 12,000 rpm for 10 min and B@PDA can be obtained. The sediment was washed two times and kept at 4 °C.
Characterizations
High-resolution transmission electron microscopy (HRTEM, Tecnai G2 F30) was used to characterize the lateral size and crystal lattice of borophene. Atomic force microscopy (AFM, Bruker, Dimension Fastscan) is used to measure the thickness of borophene. The HRTEM image was obtained with an acceleration voltage of 300 kV. AFM image was scanned in 512 pixels per line. The crystal structure of borophene was determined by X-ray diffraction (XRD) patterns using Bruker D8 system. The chemical composition was confirmed by the X-ray photoelectron spectra (XPS) using ESCALAB 250Xi equipment. UV-Vis-NIR absorbance spectrum was measured employing ultraviolet spectrophotometry (Cary 60, Agilent). An 808 nm semiconductor laser (LSR808H, Lasever Inc.) was used to irradiate the borophene for generating heat. To measure the increasing temperature, both a thermocouple and infrared thermal imaging camera (FLIR E-75) were applied.
Cell lines and reagents
The human colorectal cancer cells (HCT-116), cervical cancer cells (HeLa), lung adenocarcinoma cell line (A549) and human breast cancer cells (MCF7) were bought from American Type Culture Collection (ATCC). The cells were incubated in RPMI-1640 or Dulbecco’s Modified Eagle Medium (DMEM) supplied with 10% foetal bovine serum (FBS) and antibiotics (100 IU/mL penicillin and 100 µg/mL streptomycin), which were bought from GIBCO. Cell Counting Kit-8 and 4′, 6-Diamidino-2-phenylindole (DAPI) were bought from MCE (Monmouth Junction, NJ, USA). Enhanced chemiluminescence (ECL) kit, Mitochondrial Staining kit and Lysosome Staining Kit were obtained from BD (USA). Protease inhibitor cocktail and RIPA Lysis Buffer were bought from Sigma-Aldrich (Merck KGaA), Bicinchoninic acid (BCA) assay protein kit from EMD Millipore, and PVDF membranes from Bio Basic, Inc., Markham, ON, Canada.
Animals
Four-week-old female BALB/C nude mice aged 5–6 weeks were bought from the Guangdong Medical Experimental Animal Center (GDMLAC) and were used for in vivo imaging and therapy experiments. All the protocols for in vivo experiments were approved by the Animal Welfare and Research Ethics Committee at Shenzhen University (ID: 2017003).
References
Novoselov, K. S. et al. Electric field effect in atomically thin carbon films. Science 306, 666–669 (2004).
Ataca, C. et al. Mechanical and electronic properties of MoS2 nanoribbons and their defects. J. Phys. Chem. C 115, 3934–3941 (2011).
Wu, S. F. et al. Electrical tuning of valley magnetic moment through symmetry control in bilayer MoS2. Nat. Phys. 9, 149–153 (2013).
Xiong, F. et al. Li intercalation in MoS2: in situ observation of its dynamics and tuning optical and electrical properties. Nano Lett. 15, 6777–6784 (2015).
Lee, J., Mak, K. F. & Shan, J. Electrical control of the valley Hall effect in bilayer MoS2 transistors. Nat. Nanotechnol. 11, 421–425 (2016).
Naguib, M. et al. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 23, 4248–4253 (2011).
Xing, C. Y. et al. Two-dimensional MXene (Ti3C2)-Integrated cellulose hydrogels: toward smart three-dimensional network nanoplatforms exhibiting light-induced swelling and bimodal photothermal/chemotherapy anticancer activity. ACS Appl. Mater. Interfaces 10, 27631–27643 (2018).
Jiang, X. T. et al. Inkjet-printed MXene micro-scale devices for integrated broadband ultrafast photonics. npj 2D Mater. Appl. 3, 34 (2019).
Jiang, X. T. et al. Broadband nonlinear photonics in few-layer MXene Ti3C2Tx (T = F, O, or OH). Laser Photon. Rev. 12, 1700229 (2018).
Sun, Z. B. et al. Ultrasmall black phosphorus quantum dots: synthesis and use as photothermal agents. Angew. Chem. Int. Ed. 54, 11526–11530 (2015).
Shao, J. D. et al. Biodegradable black phosphorus-based nanospheres for in vivo photothermal cancer therapy. Nat. Commun. 7, 12967 (2016).
Xiong, S. et al. Brain-targeted delivery shuttled by black phosphorus nanostructure to treat Parkinson’s disease. Biomaterials 260, 120339 (2020).
Xie, Z. J. et al. Black phosphorus-based photothermal therapy with aCD47-mediated immune checkpoint blockade for enhanced cancer immunotherapy. Light Sci. Appl. 9, 161 (2020).
Tao, W. et al. Black phosphorus nanosheets as a robust delivery platform for cancer theranostics. Adv. Mater. 29, 1603276 (2017).
Qiu, M. et al. Novel concept of the smart NIR-light-controlled drug release of black phosphorus nanostructure for cancer therapy. Proc. Natl Acad. Sci. USA 115, 501–506 (2018).
Liang, X. et al. Photothermal cancer immunotherapy by erythrocyte membrane-coated black phosphorus formulation. J. Control. Release 296, 150–161 (2019).
Tang, X. et al. Fluorinated phosphorene: electrochemical synthesis, atomistic fluorination, and enhanced stability. Small 13, 1702739 (2017).
Qiu, M. et al. Omnipotent phosphorene: a next-generation, two-dimensional nanoplatform for multidisciplinary biomedical applications. Chem. Soc. Rev. 47, 5588–5601 (2018).
Xing, C. Y. et al. Graphene oxide/black phosphorus nanoflake aerogels with robust thermo-stability and significantly enhanced photothermal properties in air. Nanoscale 9, 8096–8101 (2017).
Tang, X. et al. Fluorination-enhanced ambient stability and electronic tolerance of black phosphorus quantum dots. Adv. Sci. 5, 1800420 (2018).
Ge, Y. Q. et al. Few-layer selenium-doped black phosphorus: synthesis, nonlinear optical properties and ultrafast photonics applications. J. Mater. Chem. C 5, 6129–6135 (2017).
Xing, C. Y. et al. Ultrasmall bismuth quantum dots: facile liquid-phase exfoliation, characterization, and application in high-performance UV-vis photodetector. ACS Photonics 5, 621–629 (2018).
Reis, F. et al. Bismuthene on a SiC substrate: a candidate for a high-temperature quantum spin hall material. Science 357, 287–290 (2017).
Lu, L. et al. All-optical switching of two continuous waves in few layer bismuthene based on spatial cross-phase modulation. ACS Photonics 4, 2852–2861 (2017).
Tao, W. et al. Two-dimensional antimonene-based photonic nanomedicine for cancer theranostics. Adv. Mater. 30, 1802061 (2018).
Ares, P. et al. Recent progress on antimonene: a new bidimensional material. Adv. Mater. 30, 1703771 (2018).
Pumera, M. & Sofer, Z. 2D monoelemental arsenene, antimonene, and bismuthene: beyond black phosphorus. Adv. Mater. 29, 1605299 (2017).
Wang, X., Song, J. & Qu, J. L. Antimonene: from experimental preparation to practical application. Angew. Chem. Int. Ed. 58, 1574–1584 (2019).
Wu, W. Z. et al. Tellurene: its physical properties, scalable nanomanufacturing, and device applications. Chem. Soc. Rev. 47, 7203–7212 (2018).
Lin, Y. et al. Two-dimensional tellurium nanosheets for photoacoustic imaging-guided photodynamic therapy. Chem. Commun. 54, 8579–8582 (2018).
Peng, J. et al. Two-dimensional tellurium nanosheets exhibiting an anomalous switchable photoresponse with thickness dependence. Angew. Chem. Int. Ed. 57, 13533–13537 (2018).
Wang, Q. S. et al. Van der waals epitaxy and photoresponse of hexagonal tellurium nanoplates on flexible mica sheets. ACS Nano 8, 7497–7505 (2014).
Wang, Y. X. et al. Field-effect transistors made from solution-grown two-dimensional tellurene. Nat. Electron. 1, 228–236 (2018).
Xie, Z. J. et al. Ultrathin 2D nonlayered tellurium nanosheets: facile liquid-phase exfoliation, characterization, and photoresponse with high performance and enhanced stability. Adv. Funct. Mater. 28, 1705833 (2018).
Cui, H. P. et al. Tellurene nanoflake-based NO2 sensors with superior sensitivity and a sub-parts-per-billion detection limit. ACS Appl. Mater. Interfaces 12, 47704–47713 (2020).
Qin, J. K. et al. Controlled growth of a large-size 2D selenium nanosheet and its electronic and optoelectronic applications. ACS Nano 11, 10222–10229 (2017).
Xing, C. Y. et al. Selenium nanosheets: 2D nonlayered selenium nanosheets: facile synthesis, photoluminescence, and ultrafast photonics. Adv. Opt. Mater. 5, 1770118 (2017).
Wu, L. M. et al. Few-layer tin sulfide: a promising black-phosphorus-analogue 2D material with exceptionally large nonlinear optical response, high stability, and applications in all-optical switching and wavelength conversion. Adv. Opt. Mater. 6, 1700985 (2018).
Xie, Z. J. et al. Revealing of the ultrafast third-order nonlinear optical response and enabled photonic application in two-dimensional tin sulfide. Photon. Res. 7, 494–502 (2019).
Xie, Z. J. et al. Black phosphorus analogue tin sulfide nanosheets: synthesis and application as near-infrared photothermal agents and drug delivery platforms for cancer therapy. J. Mater. Chem. B 6, 4747–4755 (2018).
Huang, W. C. et al. Black-phosphorus-analogue tin monosulfide: an emerging optoelectronic two-dimensional material for high-performance photodetection with improved stability under ambient/harsh conditions. J. Mater. Chem. C 6, 9582–9593 (2018).
Pei, Y. T. et al. Recent progress in the synthesis and applications of 2D metal nanosheets. Nanotechnology 30, 222001 (2019).
Xie, Z. J. et al. Biocompatible two-dimensional titanium nanosheets for multimodal imaging-guided cancer theranostics. ACS Appl. Mater. Interfaces 11, 22129–22140 (2019).
Ding, M. L. et al. Carbon capture and conversion using metal–organic frameworks and MOF-based materials. Chem. Soc. Rev. 48, 2783–2828 (2019).
Jiang, X. T. et al. Ultrathin metal–organic framework: an emerging broadband nonlinear optical material for ultrafast photonics. Adv. Opt. Mater. 6, 1800561 (2018).
Duo, Y. H. et al. Borophene-based biomedical applications: status and future challenges. Coord. Chem. Rev. 427, 213549 (2021).
Xie, Z. J. et al. Two-dimensional borophene: properties, fabrication, and promising applications. Research 2020, 2624617 (2020).
Penev, E. S. et al. Polymorphism of two-dimensional boron. Nano Lett. 12, 2441–2445 (2012).
Liu, Y. Y., Penev, E. S. & Yakobson, B. I. Probing the synthesis of two-dimensional boron by first-principles computations. Angew. Chem. Int. Ed. 52, 3156–3159 (2013).
Wu, X. J. et al. Two-dimensional boron monolayer sheets. ACS Nano 6, 7443–7453 (2012).
Zhang, Z. H. et al. Elasticity, flexibility, and ideal strength of borophenes. Adv. Funct. Mater. 27, 1605059 (2017).
Zhang, Z. H. et al. Substrate-induced nanoscale undulations of borophene on silver. Nano Lett. 16, 6622–6627 (2016).
Mannix, A. J. et al. Synthesis of borophenes: anisotropic, two-dimensional boron polymorphs. Science 350, 1513–1516 (2015).
Adamska, L. et al. First-principles investigation of borophene as a monolayer transparent conductor. J. Phys. Chem. C 122, 4037–4045 (2018).
Tai, G. A. et al. Synthesis of atomically thin boron films on copper foils. Angew. Chem. Int. Ed. 54, 15473–15477 (2015).
Tsafack, T. & Yakobson, B. I. Thermomechanical analysis of two-dimensional boron monolayers. Phys. Rev. B 93, 165434 (2016).
Liu, H. S., Gao, J. F. & Zhao, J. J. From boron cluster to two-dimensional boron sheet on Cu(111) surface: growth mechanism and hole formation. Sci. Rep. 3, 3238 (2013).
Tang, H. & Ismail-Beigi, S. Novel precursors for boron nanotubes: the competition of two-center and three-center bonding in boron sheets. Phys. Rev. Lett. 99, 115501 (2007).
Yang, X. B., Ding, Y. & Ni, J. Ab initio prediction of stable boron sheets and boron nanotubes: structure, stability, and electronic properties. Phys. Rev. B 77, 041402 (2008).
Ji, X. Y. et al. A novel top-down synthesis of ultrathin 2D boron nanosheets for multimodal imaging-guided cancer therapy. Adv. Mater. 30, 1803031 (2018).
Ranjan, P. et al. Freestanding borophene and its hybrids. Adv. Mater. 31, 1900353 (2019).
Anasori, B., Lukatskaya, M. R. & Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2, 16098 (2017).
Li, Y. X. et al. High-entropy-alloy nanoparticles with enhanced interband transitions for efficient photothermal conversion. Angew. Chem. Int. Ed. 60, 27113–27118 (2021).
Huang, W. C. et al. Recent advances in functional 2D MXene-based nanostructures for next-generation devices. Adv. Funct. Mater. 30, 2005223 (2020).
Wang, H. S. et al. Ultrahigh–current density anodes with interconnected Li metal reservoir through overlithiation of mesoporous AlF3 framework. Sci. Adv. 3, e1701301 (2017).
Djebaili, K. et al. XPS, FTIR, EDX, and XRD analysis of Al2O3 scales grown on PM2000 alloy. J. Spectrosc. 2015, 868109 (2015).
Feng, B. J. et al. Experimental realization of two-dimensional boron sheets. Nat. Chem. 8, 563–568 (2016).
Lee, C. C. et al. Peculiar bonding associated with atomic doping and hidden honeycombs in borophene. Phys. Rev. B 97, 075430 (2018).
Li, H. et al. An in situ XPS study of oxygen plasma cleaning of aluminum surfaces. Surf. Coat. Technol. 92, 171–177 (1997).
Kolmogorov, A. N. & Curtarolo, S. Prediction of different crystal structure phases in metal borides: a lithium monoboride analog to MgB2. Phys. Rev. B 73, 180501 (2006).
Liu, Q. et al. Prodrug-loaded zirconium carbide nanosheets as a novel biophotonic nanoplatform for effective treatment of cancer. Adv. Sci. 7, 2001191 (2020).
Xie, Z. J. et al. Liquid-phase exfoliation of black sesame to create a nanoplatform for in vitro photoluminescence and photothermal therapy. Nanomedicine 15, 2041–2052 (2020).
Feng, C. et al. Germanene-based theranostic materials for surgical adjuvant treatment: inhibiting tumor recurrence and wound infection. Matter 3, 127–144 (2020).
Xie, H. H. et al. Metabolizable ultrathin Bi2Se3 nanosheets in imaging-guided photothermal therapy. Small 12, 4136–4145 (2016).
Tao, W. et al. Antimonene quantum dots: synthesis and application as near-infrared photothermal agents for effective cancer therapy. Angew. Chem. Int. Ed. 56, 11896–11900 (2017).
Zeng, X. W. et al. Polydopamine-modified black phosphorous nanocapsule with enhanced stability and photothermal performance for tumor multimodal treatments. Adv. Sci. 5, 1800510 (2018).
Lee, H. et al. Mussel-inspired surface chemistry for multifunctional coatings. Science 318, 426–430 (2007).
Liu, Y. L., Ai, K. L. & Lu, L. H. Polydopamine and its derivative materials: synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 114, 5057–5115 (2014).
Liu, T. et al. Drug delivery with PEGylated MoS2 nano-sheets for combined photothermal and chemotherapy of cancer. Adv. Mater. 26, 3433–3440 (2014).
Lin, H. et al. Two-dimensional ultrathin MXene ceramic nanosheets for photothermal conversion. Nano Lett. 17, 384–391 (2017).
Yang, B. W., Chen, Y. & Shi, J. L. Reactive oxygen species (ROS)-based nanomedicine. Chem. Rev. 119, 4881–4985 (2019).
Burtenshaw, D. et al. Reactive oxygen species (ROS), intimal thickening, and subclinical atherosclerotic disease. Front. Cardiovasc. Med. 6, 89 (2019).
Sundaram, A. et al. Advanced nanomaterials for hypoxia tumor therapy: challenges and solutions. Nanoscale 12, 21497–21518 (2020).
Tee, J. K. et al. Nanoparticles’ interactions with vasculature in diseases. Chem. Soc. Rev. 48, 5381–5407 (2019).
Peng, F. et al. Nanoparticles promote in vivo breast cancer cell intravasation and extravasation by inducing endothelial leakiness. Nat. Nanotechnol. 14, 279–286 (2019).
Tee, J. K. et al. Angiopoietin-1 accelerates restoration of endothelial cell barrier integrity from nanoparticle-induced leakiness. Nanotoxicology 13, 682–700 (2019).
Sun, C. X. et al. One-pot solventless preparation of PEGylated black phosphorus nanoparticles for photoacoustic imaging and photothermal therapy of cancer. Biomaterials 91, 81–89 (2016).
Jathoul, A. P. et al. Deep in vivo photoacoustic imaging of mammalian tissues using a tyrosinase-based genetic reporter. Nat. Photon. 9, 239–246 (2015).
Sun, Z. B. et al. TiL4-coordinated black phosphorus quantum dots as an efficient contrast agent for in vivo photoacoustic imaging of cancer. Small 13, 1602896 (2017).
Xie, Z. J. et al. Emerging combination strategies with phototherapy in cancer nanomedicine. Chem. Soc. Rev. 49, 8065–8087 (2020).
Carenco, S. et al. Nanoscaled metal borides and phosphides: recent developments and perspectives. Chem. Rev. 113, 7981–8065 (2013).
Acknowledgements
This work was supported by Guangdong Scientific and Technological Project (2019B1515120043, 2020A151501612, 2021A1515220109, and 2022B1515020093), the Science and Technology Innovation Commission of Shenzhen (KCXFZ20201221173413038), the Longhua District Science and Innovation Commission Project Grants of Shenzhen (JCYJ201904). The Institute for Lasers, Photonics and Biophotonics acknowledges support from the office of the vice president for research and economic development at the University at Buffalo. The authors also acknowledge the support from Instrumental Analysis Center of Shenzhen University (Xili Campus).
Author information
Authors and Affiliations
Contributions
Z.X., Y.G., H.Z., and S.F. designed the study; Y.Z., T.F., and W.H. performed the materials fabrication and characterizations; Y.D., S.A., and H.L. performed biomedical experiments; S.F., C.L., and H.G. analyzed data; Z.X., D.L., R.G., and P.N.P. wrote the paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xie, Z., Duo, Y., Fan, T. et al. Light-induced tumor theranostics based on chemical-exfoliated borophene. Light Sci Appl 11, 324 (2022). https://doi.org/10.1038/s41377-022-00980-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41377-022-00980-9