Abstract
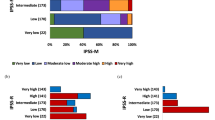
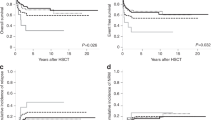
The Molecular International Prognostic Scoring System (IPSS-M) is a novel risk stratification model for myelodysplastic syndromes (MDS) that builds on the IPSS and IPSS-R by incorporating mutational data. The model showed improved prognostic accuracy over the IPSS-R across three endpoints: overall survival (OS), leukemia-free survival (LFS) and leukemic transformation. This study aimed to validate the findings of the original in a large cohort of MDS patients, as well as assess its validity in therapy-related and hypoplastic MDS. We retrospectively reviewed clinical, cytogenetic and molecular data for 2355 MDS patients treated at the Moffitt Cancer Center. Correlative analysis between IPSS-R and mean IPSS-M scores and outcome predictions was performed on LFS, OS and leukemic transformation. Using the IPSS-M, patients were classified as Very Low (4%), Low (24%), Moderate-Low (14%), Moderate-High (11%), High (19%) and Very-High risk (28%). Median OS was 11.7, 7.1, 4.4, 3.1, 2.3, and 1.3 years from VL to VH risk subgroups. Median LFS was 12.3, 6.9, 3.6, 2.2, 1.4, and 0.5 years respectively. For patients with t-MDS and h-MDS the model retained its prognostic accuracy. Generalized use of this tool will likely result in more accurate prognostic assessment and optimize therapeutic decision-making in MDS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the paper and/or its supplementary materials.
References
Aguirre LE, Komrokji R, Padron E. It is time to shift the treatment paradigm in myelodysplastic syndromes: A focus on novel developments and current investigational approaches exploring combinatorial therapy in high-risk MDS. Best Pr Res Clin Haematol. 2021;34:101325.
Ogawa S. Genetics of MDS. Blood. 2019;133:1049–59.
Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–88.
Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Solé F, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454–65.
Bejar R. Clinical and genetic predictors of prognosis in myelodysplastic syndromes. Haematologica 2014;99:956–64.
Malcovati L, Karimi M, Papaemmanuil E, Ambaglio I, Jädersten M, Jansson M, et al. SF3B1 mutation identifies a distinct subset of myelodysplastic syndrome with ring sideroblasts. Blood. 2015;126:233–41.
Malcovati L, Stevenson K, Papaemmanuil E, Neuberg D, Bejar R, Boultwood J, et al. SF3B1-mutant MDS as a distinct disease subtype: a proposal from the International Working Group for the Prognosis of MDS. Blood. 2020;136:157–70.
Papaemmanuil E, Gerstung M, Malcovati L, Tauro S, Gundem G, Van Loo P, et al. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood. 2013;122:3616–27.
Haferlach T, Nagata Y, Grossmann V, Okuno Y, Bacher U, Nagae G, et al. Landscape of genetic lesions in 944 patients with myelodysplastic syndromes. Leukemia. 2014;28:241–7.
Nazha A, Al-Issa K, Hamilton BK, Radivoyevitch T, Gerds AT, Mukherjee S, et al. Adding molecular data to prognostic models can improve predictive power in treated patients with myelodysplastic syndromes. Leukemia. 2017;31:2848–50.
Bersanelli M, Travaglino E, Meggendorfer M, Matteuzzi T, Sala C, Mosca E, et al. Classification and personalized prognostic assessment on the basis of clinical and genomic features in myelodysplastic syndromes. J Clin Oncol. 2021;39:1223–33.
Nazha A, Komrokji R, Meggendorfer M, Jia X, Radakovich N, Shreve J, et al. Personalized prediction model to risk stratify patients with myelodysplastic syndromes. J Clin Oncol. 2021;39:3737–46.
Bernard E, Tuechler H, Greenberg PL, Hasserjian RP, Arango JE, Nannya Y, et al. Molecular International Prognostic Scoring System for Myelodysplastic Syndromes. NEJM Evid. 2022;1:1–14.
Kuendgen A, Nomdedeu M, Tuechler H, Garcia-Manero G, Komrokji RS, Sekeres MA, et al. Therapy-related myelodysplastic syndromes deserve specific diagnostic sub-classification and risk-stratification-an approach to classification of patients with t-MDS. Leukemia 2021;35:835–49.
Mishra A, Corrales-Yepez M, Al-Ali N, Kharfan-Dabaja M, Padron E, Zhang L, et al. Validation of the revised International Prognostic Scoring System in treated patients with myelodysplastic syndromes. Am J Hematol. 2013;88:566–70.
Bono E, McLornan D, Travaglino E, Gandhi S, Gallì A, Khan AA, et al. Clinical, histopathological and molecular characterization of hypoplastic myelodysplastic syndrome. Leukemia 2019;33:2495–505.
Fattizzo B, Serpenti F, Barcellini W, Caprioli C. Hypoplastic Myelodysplastic Syndromes: Just an Overlap Syndrome? Cancers (Basel). 2021;13:132.
Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022;36:1703–19.
Platzbecker U. Treatment of MDS. Blood. 2019;133:1096–107.
Arber DA, Orazi A, Hasserjian RP, Borowitz MJ, Calvo KR, Kvasnicka HM, et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: integrating morphologic, clinical, and genomic data. Blood 2022;140:1200–28.
Bernard E, Nannya Y, Hasserjian RP, Devlin SM, Tuechler H, Medina-Martinez JS, et al. Implications of TP53 allelic state for genome stability, clinical presentation and outcomes in myelodysplastic syndromes. Nat Med. 2020;26:1549–56.
Jumniensuk C, Nobori A, Lee T, Senaratne TN, Rao D, Pullarkat S. Concordance of Peripheral Blood and Bone Marrow Next-Generation Sequencing in Hematologic Neoplasms. Adv Hematol. 2022;2022:8091746.
Lucas F, Michaels PD, Wang D, Kim AS. Mutational analysis of hematologic neoplasms in 164 paired peripheral blood and bone marrow samples by next-generation sequencing. Blood Adv. 2020;4:4362–5.
Acknowledgements
Parts of this paper will be presented in preliminary form as three separate abstracts at the 2022 64th ASH Annual Meeting and Exposition as an oral presentation and three tow posters. The investigators would like to extend their sincerest gratitude to Dr Elsa Bernard and the Elli Papaemmanuil lab at Memorial Sloan Kettering Cancer Center for providing syntax code and additional support to facilitate batch-calculation analysis.
Author information
Authors and Affiliations
Contributions
(I) Conception and design: RK, LA. (II) Administrative support: RK, NA. (III) Provision of study materials or patients: RK, LA, DS, JL, KS, EP, NA, AK. (IV) Collection and assembly of data: NA, RK, LA. (V) Data analysis and interpretation: RK, LA, NA, OC. (VI) Paper writing: LA, RK, DS. (VII) Final approval of paper: All authors.
Corresponding authors
Ethics declarations
Competing interests
All authors have completed the ICMJE uniform disclosure form. T-V: Abbvie: Consultancy; Novartis: Consultancy; Jazz: Consultancy, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; CTI: Consultancy, Speakers Bureau; BMS: Consultancy, Speakers Bureau. AK: Protagonist: Other: Research Support; Imago Biosciences: Consultancy, Honoraria, Speakers Bureau; Incyte: Consultancy, Honoraria, Speakers Bureau; Blueprint Medicines Corporation: Consultancy, Honoraria, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau; Abbvie: Consultancy, Honoraria, Speakers Bureau; GSK - Sierra Oncology: Consultancy, Honoraria, Other: Research Support, Speakers Bureau; Prelude Pharmaceuticals: Other: Research Support; BMS: Consultancy, Honoraria, Other: Research Support, Speakers Bureau; Morphosys: Other: Research Support; Pharmaessentia: Consultancy, Honoraria, Speakers Bureau; CTI Biopharma: Consultancy, Honoraria, Speakers Bureau. KS: Mablytics: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity’s Board of Directors or advisory committees, Research Funding; Curis: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees; berGenBio: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees; Syntrix Pharmaceuticals: Research Funding; Incyte: Research Funding; AROG: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees; Gilead Sciences, Inc.: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees; Astellas: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees. JEL: Novartis: Consultancy; Jasper Therapeutics: Consultancy; Dedham Group: Consultancy; Boxer Capital: Consultancy; Dava Oncology: Consultancy; Syntrix Pharmaceuticals: Research Funding; Astellas: Consultancy; Agios/Servio: Consultancy; Jazz: Consultancy; BerGenBio: Consultancy; Millenium Pharma/Takeda: Consultancy; ElevateBio Management: Consultancy; Daiichi Sankyo: Consultancy; Celgene/BMS: Research Funding; AbbVie: Consultancy; Servier: Consultancy. EP: Incyte: Research Funding; Kura: Research Funding; Stemline: Honoraria; Taiho: Honoraria; Blueprint: Honoraria; Syntrix Pharmaceuticals: Research Funding; BMS: Research Funding. DAS: Syntrix Pharmaceuticals: Research Funding; Nemucore: Membership on an entity’s Board of Directors or advisory committees; Lixte: Patents & Royalties: LB-100; Magenta: Consultancy; Novartis: Consultancy, Membership on an entity’s Board of Directors or advisory committees; Intellia: Membership on an entity’s Board of Directors or advisory committees; Syndax: Membership on an entity’s Board of Directors or advisory committees; Kite: Membership on an entity’s Board of Directors or advisory committees; Incyte: Speakers Bureau; Shattuck Labs: Membership on an entity’s Board of Directors or advisory committees; BMS: Membership on an entity’s Board of Directors or advisory committees, Speakers Bureau; Aprea: Membership on an entity’s Board of Directors or advisory committees, Research Funding; Agios: Membership on an entity’s Board of Directors or advisory committees; AbbVie: Membership on an entity’s Board of Directors or advisory committees; Takeda: Consultancy. RSK: Jazz: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Speakers Bureau; Servio: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Speakers Bureau; CTI biopharma: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Speakers Bureau; PharmaEssentia: Honoraria, Other, Speakers Bureau; BMS: Honoraria, Membership on an entity’s Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity’s Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity’s Board of Directors or advisory committees; Geron: Honoraria, Membership on an entity’s Board of Directors or advisory committees; Taiho: Honoraria, Membership on an entity’s Board of Directors or advisory committees. All other authors have no competing interests to declare.
Ethics approval
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board and individual consent for this retrospective analysis was waived.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aguirre, L.E., Al Ali, N., Sallman, D.A. et al. Assessment and validation of the molecular international prognostic scoring system for myelodysplastic syndromes. Leukemia 37, 1530–1539 (2023). https://doi.org/10.1038/s41375-023-01910-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-023-01910-3
This article is cited by
-
Clinical impact of genetic alterations including germline DDX41 mutations in MDS/low-blast count AML patients treated with azacitidine-based regimens
Leukemia (2024)
-
Treatment of Myelodysplastic Syndromes for Older Patients: Current State of Science, Challenges, and Opportunities
Current Hematologic Malignancy Reports (2024)