Abstract
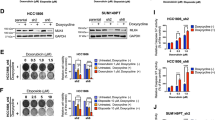
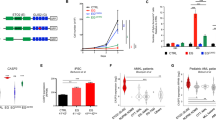
DLBCL is the most common lymphoma with high tumor heterogeneity. Treatment refractoriness and relapse from R-CHOP therapy in patients remain a clinical problem. Activation of the non-canonical NF-κB pathway is associated with R-CHOP resistance. However, downstream targets of non-canonical NF-κB mediating R-CHOP-induced resistance remains uncharacterized. Here, we identify the common mechanisms underlying both intrinsic and acquired resistance that are induced by doxorubicin, the main cytotoxic component of R-CHOP. We performed global transcriptomic analysis of (1) a panel of resistant versus sensitive and (2) isogenic acquired doxorubicin-resistant DLBCL cell lines following short and chronic exposure to doxorubicin respectively. Doxorubicin-induced stress in resistant cells activates a distinct transcriptional signature that is enriched in metabolic reprogramming and oncogenic signalling. Selective and sustained activation of non-canonical NF-κB signalling in these resistant cells exacerbated their survival by augmenting glycolysis. In response to doxorubicin, p52-RelB complexes transcriptionally activated multiple glycolytic regulators with prognostic significance through increased recruitment at their gene promoters. Targeting p52-RelB and their targets in resistant cells increased doxorubicin sensitivity in vitro and in vivo. Collectively, our study uncovered novel molecular drivers of doxorubicin-induced resistance that are regulated by non-canonical NF-κB pathway. We reveal new avenues of therapeutic targeting for R-CHOP-treated refractory/relapsed DLBCL patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The RNA-seq datasets generated during and/or analysed during the current study had been deposited in the GEO (Gene Expression Omnibus) database under the accession number GSE215900.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49.
Susanibar-Adaniya S, Barta SK. 2021 Update on Diffuse large B cell lymphoma: A review of current data and potential applications on risk stratification and management. Am J Hematol. 2021;96:617–29.
Wu JQ, Song YP, Su LP, Zhang MZ, Li W, Hu Y, et al. Three-year Follow-up on the Safety and Effectiveness of Rituximab Plus Chemotherapy as First-Line Treatment of Diffuse Large B-Cell Lymphoma and Follicular Lymphoma in Real-World Clinical Settings in China: A Prospective, Multicenter, Noninterventional Study. Chin Med J (Engl). 2018;131:1767–75.
He MY, Kridel R. Treatment resistance in diffuse large B-cell lymphoma. Leukemia. 2021;35:2151–65.
Palmer AC, Chidley C, Sorger PK. A curative combination cancer therapy achieves high fractional cell killing through low cross-resistance and drug additivity. Elife 2019;8:e50036.
Nowakowski GS, Czuczman MSABC, GCB, and Double-Hit Diffuse Large B-Cell Lymphoma: Does Subtype Make a Difference in Therapy Selection? Am Soc Clin Oncol Educ Book 2015: e449–57.
Erttmann R, Erb N, Steinhoff A, Landbeck G. Pharmacokinetics of doxorubicin in man: dose and schedule dependence. J Cancer Res Clin Oncol. 1988;114:509–13.
Yang W, Soares J, Greninger P, Edelman EJ, Lightfoot H, Forbes S, et al. Genomics of Drug Sensitivity in Cancer (GDSC): a resource for therapeutic biomarker discovery in cancer cells. Nucl Acids Res. 2013;41:D955–961.
Bonner WM, Redon CE, Dickey JS, Nakamura AJ, Sedelnikova OA, Solier S, et al. GammaH2AX and cancer. Nat Rev Cancer. 2008;8:957–67.
Bieging KT, Mello SS, Attardi LD. Unravelling mechanisms of p53-mediated tumour suppression. Nat Rev Cancer. 2014;14:359–70.
Chen X, Chen S, Yu D. Metabolic Reprogramming of Chemoresistant Cancer Cells and the Potential Significance of Metabolic Regulation in the Reversal of Cancer Chemoresistance. Metabolites 2020;10:289.
Zhong Z, Virshup DM. Wnt Signaling and Drug Resistance in Cancer. Mol Pharm. 2020;97:72–89.
Flahaut M, Meier R, Coulon A, Nardou KA, Niggli FK, Martinet D, et al. The Wnt receptor FZD1 mediates chemoresistance in neuroblastoma through activation of the Wnt/beta-catenin pathway. Oncogene. 2009;28:2245–56.
Yuniati L, Scheijen B, van der Meer LT, van Leeuwen FN. Tumor suppressors BTG1 and BTG2: Beyond growth control. J Cell Physiol. 2019;234:5379–89.
Tamura RE, de Vasconcellos JF, Sarkar D, Libermann TA, Fisher PB, Zerbini LF. GADD45 proteins: central players in tumorigenesis. Curr Mol Med. 2012;12:634–51.
Bensaad K, Tsuruta A, Selak MA, Vidal MN, Nakano K, Bartrons R, et al. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126:107–20.
Xu H, Yan M, Patra J, Natrajan R, Yan Y, Swagemakers S, et al. Enhanced RAD21 cohesin expression confers poor prognosis and resistance to chemotherapy in high grade luminal, basal and HER2 breast cancers. Breast Cancer Res. 2011;13:R9.
Yu J, Zhou J, Xu F, Bai W, Zhang W. High expression of Aurora-B is correlated with poor prognosis and drug resistance in non-small cell lung cancer. Int J Biol Markers. 2018;33:215–21.
Zhu Y, Li K, Zhang J, Wang L, Sheng L, Yan L. Inhibition of CDK1 Reverses the Resistance of 5-Fu in Colorectal Cancer. Cancer Manag Res. 2020;12:11271–83.
Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, et al. Addendum: The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2019;565:E5–E6.
Zhou Y, Tozzi F, Chen J, Fan F, Xia L, Wang J, et al. Intracellular ATP levels are a pivotal determinant of chemoresistance in colon cancer cells. Cancer Res. 2012;72:304–14.
Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33.
Xu Y, Fang F, St Clair DK, Sompol P, Josson S, St Clair WH. SN52, a novel nuclear factor-kappaB inhibitor, blocks nuclear import of RelB:p52 dimer and sensitizes prostate cancer cells to ionizing radiation. Mol Cancer Ther. 2008;7:2367–76.
Zhao B, Barrera LA, Ersing I, Willox B, Schmidt SC, Greenfeld H, et al. The NF-kappaB genomic landscape in lymphoblastoid B cells. Cell Rep. 2014;8:1595–606.
de Oliveira KA, Kaergel E, Heinig M, Fontaine JF, Patone G, Muro EM, et al. A roadmap of constitutive NF-kappaB activity in Hodgkin lymphoma: Dominant roles of p50 and p52 revealed by genome-wide analyses. Genome Med. 2016;8:28.
Li J, He Y, Tan Z, Lu J, Li L, Song X, et al. Wild-type IDH2 promotes the Warburg effect and tumor growth through HIF1alpha in lung cancer. Theranostics. 2018;8:4050–61.
Zhao J, Li J, Fan TWM, Hou SX. Glycolytic reprogramming through PCK2 regulates tumor initiation of prostate cancer cells. Oncotarget. 2017;8:83602–18.
Jiang Y, He R, Jiang Y, Liu D, Tao L, Yang M, et al. Transcription factor NFAT5 contributes to the glycolytic phenotype rewiring and pancreatic cancer progression via transcription of PGK1. Cell Death Dis. 2019;10:948.
Saumet A, Vetter G, Bouttier M, Antoine E, Roubert C, Orsetti B, et al. Estrogen and retinoic acid antagonistically regulate several microRNA genes to control aerobic glycolysis in breast cancer cells. Mol Biosyst. 2012;8:3242–53.
Liu W, Sun Y, Ge W, Zhang F, Gan L, Zhu Y, et al. DIA-Based Proteomics Identifies IDH2 as a Targetable Regulator of Acquired Drug Resistance in Chronic Myeloid Leukemia. Mol Cell Proteom. 2022;21:100187.
Vaeth M, Feske S. NFAT control of immune function: New Frontiers for an Abiding Trooper. F1000Res. 2018;7:260.
Bruce JIE. Metabolic regulation of the PMCA: Role in cell death and survival. Cell Calcium. 2018;69:28–36.
Lovas A, Weidemann A, Albrecht D, Wiechert L, Weih D, Weih F. p100 Deficiency is insufficient for full activation of the alternative NF-kappaB pathway: TNF cooperates with p52-RelB in target gene transcription. PLoS One. 2012;7:e42741.
Yang Y, Kelly P, Shaffer AL 3rd, Schmitz R, Yoo HM, Liu X, et al. Targeting Non-proteolytic Protein Ubiquitination for the Treatment of Diffuse Large B Cell Lymphoma. Cancer Cell. 2016;29:494–507.
Simpson L, Ansell SM, Colgan JP, Habermann TM, Inwards DJ, Ristow KM, et al. Effectiveness of second line salvage chemotherapy with ifosfamide, carboplatin, and etoposide in patients with relapsed diffuse large B-cell lymphoma not responding to cis-platinum, cytosine arabinoside, and dexamethasone. Leuk Lymphoma. 2007;48:1332–7.
Sobrevilla-Calvo P, Vargas-Hernandez L, Cortes-Padilla D, Labardini-Mendez JR, Oñate-Ocaña LF. Dexamethasone, etoposide and cisplatin (DEP) as second line chemotherapy in patients with diffuse large B cell lymphoma (DLBCL). J Clin Oncol. 2005;23:6723.
Wang X, Yan J, Shen B, Wei G. Integrated Chromatin Accessibility and Transcriptome Landscapes of Doxorubicin-Resistant Breast Cancer Cells. Front Cell Dev Biol. 2021;9:708066.
Park HY, Lee SB, Yoo HY, Kim SJ, Kim WS, Kim JI, et al. Whole-exome and transcriptome sequencing of refractory diffuse large B-cell lymphoma. Oncotarget. 2016;7:86433–45.
Pham LV, Tamayo AT, Li C, Lee J, Fayad L, Ford RJ. Chemo-Resistance in Diffuse Large Cell Lymphoma: Novel Drug Combinations Targeting NFAT/NF-Kb Growth/Survival/Chemo-Resistance Signaling Pathways in Validated Novel Experimental Systems. Blood. 2011;118:1428.
Eluard B, Nuan-Aliman S, Faumont N, Collares D, Bordereaux D, Montagne A, et al. The alternative RelB NF-kappaB subunit is a novel critical player in diffuse large B-cell lymphoma. Blood. 2022;139:384–98.
McGuirk S, Audet-Delage Y, Annis MG, Xue Y, Vernier M, Zhao, K et al. Resistance to different anthracycline chemotherapeutics elicits distinct and actionable primary metabolic dependencies in breast cancer. Elife 2021;10:e65150.
Nuan-Aliman S, Bordereaux D, Thieblemont C, Baud V. The Alternative RelB NF-kB Subunit Exerts a Critical Survival Function upon Metabolic Stress in Diffuse Large B-Cell Lymphoma-Derived Cells. Biomedicines 2022;10:348.
Gorini S, De Angelis A, Berrino L, Malara N, Rosano G, Ferraro E. Chemotherapeutic Drugs and Mitochondrial Dysfunction: Focus on Doxorubicin, Trastuzumab, and Sunitinib. Oxid Med Cell Longev. 2018;2018:7582730.
Jing Z, Gao J, Li J, Niu F, Tian L, Nan P, et al. Acetylation-induced PCK isoenzyme transition promotes metabolic adaption of liver cancer to systemic therapy. Cancer Lett. 2021;519:46–62.
Cole AJ, Iyengar M, Panesso-Gomez S, O’Hayer P, Chan D, Delgoffe GM, et al. NFATC4 promotes quiescence and chemotherapy resistance in ovarian cancer. JCI Insight. 2020;5:e131486.
de Sousa EMF, Vermeulen L. Wnt Signaling in Cancer Stem Cell Biology. Cancers (Basel) 2016; 8:60.
El-Sahli S, Xie Y, Wang L, Liu S. Wnt Signaling in Cancer Metabolism and Immunity. Cancers (Basel) 2019;11:904.
Du Q, Geller DA. Cross-Regulation Between Wnt and NF-kappaB Signaling. Pathw Immunopathol Dis Ther. 2010;1:155–81.
Schwitalla S, Fingerle AA, Cammareri P, Nebelsiek T, Goktuna SI, Ziegler PK, et al. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell. 2013;152:25–38.
Acknowledgements
We thank Hui Fen Kerry May Lim and Dachuan Huang from National Cancer Centre, Singapore for establishing the PDX model. This study is funded by the National Research Foundation (NRF) Singapore, under its Singapore NRF Fellowship (NRF-NRFF2018-04). In addition, we thank the Nanyang Assistant Professorship (NAP) Start-up-grant to Y.L. lab and National Medical Research Council (NMRC-OFLCG-18May0028), Tanoto Foundation and Ling Foundation for their support.
Author information
Authors and Affiliations
Contributions
YL conceptualized and supervised the study. YL and SKL planned and devised the experiments. SKL, SL and AB performed all molecular and cell biology experiments. CCP and VV performed all bioinformatics analyses and data visualization. STL and CKO contributed the PDX model and BHP performed the PDX ex vivo experiments. ST and SKL conducted the tumor xenograft studies. SKL analysed the data and JQL reviewed all statistical analyses. ADJ provided feedback on the study design. SKL wrote the manuscript and YL edited it.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lim, S.K., Peng, C.C., Low, S. et al. Sustained activation of non-canonical NF-κB signalling drives glycolytic reprogramming in doxorubicin-resistant DLBCL. Leukemia 37, 441–452 (2023). https://doi.org/10.1038/s41375-022-01769-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-022-01769-w
This article is cited by
-
NF-κB in biology and targeted therapy: new insights and translational implications
Signal Transduction and Targeted Therapy (2024)
-
Aberrant non-canonical NF-κB signalling reprograms the epigenome landscape to drive oncogenic transcriptomes in multiple myeloma
Nature Communications (2024)
-
NF-κB/p52 augments ETS1 binding genome-wide to promote glioma progression
Communications Biology (2023)