Abstract
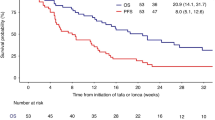
Persistence or recurrence of large B-cell lymphoma after CD19-CAR-T is common, yet data guiding management are limited. We describe outcomes and features following CAR-T treatment failure. Of 305 adults who received CD19-CAR-T, 182 experienced disease recurrence or progression (1-year cumulative incidence 63% [95%CI: 57–69]). Of 52 post-CAR-T biopsies evaluated by flow cytometry, 49 (94%) expressed CD19. Subsequent anti-cancer treatment was administered in 135/182 (74%) patients with CAR-T treatment failure. Median OS from the first post-CAR-T treatment was 8 months (95%CI 5.6–11.0). Polatuzumab-, standard chemotherapy-, and lenalidomide-based treatments were the most common approaches after CAR-T. No complete responses (CRs) were observed with conventional chemotherapy, while CR rates exceeding 30% were seen following polatuzumab- or lenalidomide-based therapies. Factors associated with poor OS among patients treated post-CAR-T were pre-CAR-T bulky disease (HR 2.27 [1.10–4.72]), lack of response to CAR-T (2.33 [1.02–5.29]), age >65 years (HR 2.65 [1.49–4.73]) and elevated LDH at post-CAR-T treatment (HR 2.95 [1.61–5.38]). The presence of ≥2 of these factors was associated with inferior OS compared to ≤1 (56% vs. 19%). In this largest analysis to date of patients who progressed or relapsed after CD19-CAR-T, survival is poor, though novel agents such as polatuzumab and lenalidomide may have hold promise.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to protect study participant privacy but are available from the corresponding author upon reasonable request and approval by MSK IRB
References
Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–44.
Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396:839–52.
Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380:45–56.
Sauter CS, Senechal B, Riviere I, Ni A, Bernal Y, Wang X, et al. CD19 CAR T cells following autologous transplantation in poor-risk relapsed and refractory B-cell non-Hodgkin lymphoma. Blood. 2019;134:626–35.
Ortíz-Maldonado V, Rives S, Castellà M, Alonso-Saladrigues A, Benítez-Ribas D, Caballero-Baños M, et al. CART19-BE-01: a multicenter trial of ARI-0001 cell therapy in patients with CD19 + relapsed/refractory malignancies. Mol Ther. 2021;29:636–44.
Kedmi M, Shouval R, Fried S, Bomze D, Fein J, Cohen Z, et al. Point-of-care anti-CD19 CAR T-cells for treatment of relapsed and refractory aggressive B-cell lymphoma. Transplant Cell Ther. 2022;28:251–7.
Itzhaki O, Jacoby E, Nissani A, Levi M, Nagler A, Kubi A, et al. Head-to-head comparison of in-house produced CD19 CAR-T cell in ALL and NHL patients. J Immunother Cancer. 2020;8:e000148.
Cappell KM, Sherry RM, Yang JC, Goff SL, Vanasse DA, McIntyre L, et al. Long-term follow-up of Anti-CD19 chimeric antigen receptor T-cell therapy. J Clin Oncol. 2020;38:3805–15.
Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20:31–42.
Vercellino L, Di Blasi R, Kanoun S, Tessoulin B, Rossi C, D’Aveni-Piney M, et al. Predictive factors of early progression after CAR T-cell therapy in relapsed/refractory diffuse large B-cell lymphoma. Blood Adv. 2020;4:5607–15.
Chow VA, Gopal AK, Maloney DG, Turtle CJ, Smith SD, Ujjani CS, et al. Outcomes of patients with large B-cell lymphomas and progressive disease following CD19-specific CAR T-cell therapy. Am J Hematol. 2019;94:E209–E213.
Nastoupil LJ, Jain MD, Feng L, Spiegel JY, Ghobadi A, Lin Y, et al. Standard-of-care axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma: results from the US lymphoma CAR T consortium. J Clin Oncol. 2020;38:3119–28.
Spiegel JY, Dahiya S, Jain MD, Tamaresis J, Nastoupil LJ, Jacobs MT, et al. Outcomes of patients with large B-cell lymphoma progressing after axicabtagene ciloleucel therapy. Blood, J Am Soc Hematol. 2021;137:1832–5.
Salles G, Duell J, Gonzalez Barca E, Tournilhac O, Jurczak W, Liberati AM, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. 2020;21:978–88.
Tilly H, Morschhauser F, Bartlett NL, Mehta A, Salles G, Haioun C, et al. Polatuzumab vedotin in combination with immunochemotherapy in patients with previously untreated diffuse large B-cell lymphoma: an open-label, non-randomised, phase 1b-2 study. Lancet Oncol. 2019;20:998–1010.
Kalakonda N, Maerevoet M, Cavallo F, Follows G, Goy A, Vermaat JSP, et al. Selinexor in patients with relapsed or refractory diffuse large B-cell lymphoma (SADAL): a single-arm, multinational, multicentre, open-label, phase 2 trial. Lancet Haematol. 2020;7:e511–e522.
Hamadani M, Radford J, Carlo-Stella C, Caimi PF, Reid E, O’Connor OA, et al. Final results of a phase 1 study of loncastuximab tesirine in relapsed/refractory B-cell non-Hodgkin lymphoma. Blood. 2021;137:2634–45.
Logue JM, Chavez JC. How to sequence therapies in diffuse large B-cell lymphoma post-CAR-T cell failure. Curr Treat Options Oncol. 2021;22:112. 2021/10/26
Byrne M, Oluwole OO, Savani B, Majhail NS, Hill BT, Locke FL. Understanding and managing large B cell lymphoma relapses after chimeric antigen receptor T cell therapy. Biol Blood Marrow Transpl. 2019;25:e344–e351.
Imber BS, Sadelain M, DeSelm C, Batlevi C, Brentjens RJ, Dahi PB, et al. Early experience using salvage radiotherapy for relapsed/refractory non‐Hodgkin lymphomas after CD19 chimeric antigen receptor (CAR) T cell therapy. Br J Haematol. 2020;190:45–51.
Liebers N, Duell J, Fitzgerald D, Kerkhoff A, Noerenberg D, Kaebisch E, et al. Polatuzumab vedotin as a salvage and bridging treatment in relapsed or refractory large B-cell lymphomas. Blood Adv. 2021;5:2707–16.
Gouni S, Rosenthal AC, Crombie JL, Ip A, Kamdar M, Hess B, et al. A multicenter retrospective study of polatuzumab vedotin in patients with large B-cell lymphoma after CAR T-cell therapy. Blood Adv. 2022;6:2757–62.
Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208.
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–68.
Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transpl. 2019;25:625–38.
Palomba ML, Qualls D, Monette S, Sethi S, Dogan A, Roshal M, et al. CD19-directed chimeric antigen receptor T cell therapy in Waldenstrom macroglobulinemia: a preclinical model and initial clinical experience. J Immunother Cancer. 2022;10:e004128.
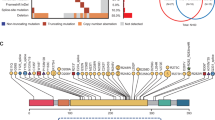
Orlando EJ, Han X, Tribouley C, Wood PA, Leary RJ, Riester M, et al. Genetic mechanisms of target antigen loss in CAR19 therapy of acute lymphoblastic leukemia. Nat Med. 2018;24:1504–6.
Dourthe M-E, Rabian F, Yakouben K, Chevillon F, Cabannes-Hamy A, Méchinaud F, et al. Determinants of CD19-positive vs CD19-negative relapse after tisagenlecleucel for B-cell acute lymphoblastic leukemia. Leukemia. 2021;35:3383–93. 2021/12/01
Larson RC, Maus MV. Recent advances and discoveries in the mechanisms and functions of CAR T cells. Nat Rev Cancer. 2021;21:145–61.
Hay KA, Gauthier J, Hirayama AV, Voutsinas JM, Wu Q, Li D, et al. Factors associated with durable EFS in adult B-cell ALL patients achieving MRD-negative CR after CD19 CAR T-cell therapy. Blood. 2019;133:1652–63.
Plaks V, Rossi JM, Chou J, Wang LH, Poddar S, Han GC, et al. CD19 target evasion as a mechanism of relapse in large B-cell lymphoma treated with axicabtagene ciloleucel. Blood. 2021;138:1081–5.
Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Reagan PM, Miklos DB, et al. Comparison of 2-year outcomes with CAR T cells (ZUMA-1) vs salvage chemotherapy in refractory large B-cell lymphoma. Blood Adv. 2021;5:4149–55.
Garcia-Recio M, Wudhikarn K, Pennisi M, Alonso-Trillo R, Flynn J, Shouval R, et al. The International Prognostic Index is associated with outcomes in diffuse Large B cell lymphoma after chimeric antigen receptor T cell therapy. Transpl Cell Ther. 2021;27:233–40.
Gauthier J, Bezerra ED, Hirayama AV, Fiorenza S, Sheih A, Chou CK, et al. Factors associated with outcomes after a second CD19-targeted CAR T-cell infusion for refractory B-cell malignancies. Blood. 2021;137:323–35.
Zhang Y, Wang Y, Liu Y, Tong C, Wang C, Guo Y, et al. Long-term activity of tandem CD19/CD20 CAR therapy in refractory/relapsed B-cell lymphoma: a single-arm, phase 1-2 trial. Leukemia. 2021;36:189–96.
Locke FL, Miklos DB, Jacobson CA, Perales M-A, Kersten M-J, Oluwole OO, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386:640–54.
Bishop MR, Dickinson M, Purtill D, Barba P, Santoro A, Hamad N, et al. Second-line tisagenlecleucel or standard care in aggressive B-cell lymphoma. N Engl J Med. 2022;386:629–39.
Neelapu SS, Dickinson M, Munoz J, Ulrickson ML, Thieblemont C, Oluwole OO, et al. Axicabtagene ciloleucel as first-line therapy in high-risk large B-cell lymphoma: the phase 2 ZUMA-12 trial. Nat Med. 2022;28:735–42.
Works M, Soni N, Hauskins C, Sierra C, Baturevych A, Jones JC, et al. Anti–B-cell maturation antigen chimeric antigen receptor T cell function against multiple myeloma is enhanced in the presence of lenalidomide. Mol Cancer Ther. 2019;18:2246–57.
Otáhal P, Průková D, Král V, Fabry M, Vočková P, Latečková L, et al. Lenalidomide enhances antitumor functions of chimeric antigen receptor-modified T cells. Oncoimmunology. 2016;5:e1115940.
Kuramitsu S, Ohno M, Ohka F, Shiina S, Yamamichi A, Kato A, et al. Lenalidomide enhances the function of chimeric antigen receptor T cells against the epidermal growth factor receptor variant III by enhancing immune synapses. Cancer Gene Ther. 2015;22:487–95.
Thieblemont C, Chevret S, Allain V, Di Blasi R, Morin F, Vercellino L, et al. Lenalidomide enhance CAR T-cells response in patients with refractory/relapsed large B cell lymphoma experiencing progression after infusion. Abstract from the 62nd American Society of Hematology Annual Meeting and Exposition. Blood. 2020;136. Poster 1115.
Van Den Neste E, Schmitz N, Mounier N, Gill D, Linch D, Trneny M, et al. Outcome of patients with relapsed diffuse large B-cell lymphoma who fail second-line salvage regimens in the International CORAL study. Bone Marrow Transpl. 2016;51:51–7.
Acknowledgements
This research was supported in part by the Memorial Sloan Kettering Cancer Center Core grant (P30 CA008748) from the National Institutes of Health/National Cancer Institute. RS was supported by the American Society of Transplantation and Cellular Therapy New Investigator Award, the American Society of Hematology Fellow Scholar Award, a grant from the Long Island Sound Chapter, Swim Across America, the Robert Hirschhorn Award, and the Memorial Sloan Kettering Steven Greenberg Lymphoma Research Award. AAT was supported by a grant from the Alfonso Martín Escudero. JAF was supported by an HONORS Award from the American Society of Hematology.
Author information
Authors and Affiliations
Contributions
Conception and design: RS, AAT, JAF, M-AP. Provision of study materials or patients: RJL, MS, CB, MB, PBD, ID, SG, BSI, EJ, MK, AN, LP, GS, CS, NS-T, AS, MR, JY, RY, GS, AA, M-AP. Collection and assembly of data: AAT, JAF, SF, WBF, TA, AA, NS, EF, RJL, MS, MR, RS. Data analysis and interpretation: JF, SD, RS, AAT, JAF, M-AP. Manuscript writing: All authors. Final approval of manuscript: All authors. Accountable for all aspects of the work: All authors.
Corresponding authors
Ethics declarations
Competing interests
RS: Medexus, Consultancy; PBD: Kite/Gilead, Advisory board; SAG: Actinnum, Membership on an entity’s Board of Directors or advisory committees; CELGENE, Membership on an entity’s Board of Directors or advisory committees; BMS., Membership on an entity’s Board of Directors or advisory committees; SANOFI, Membership on an entity’s Board of Directors or advisory committees; AMGEN, Membership on an entity’s Board of Directors or advisory committees; PFIZER, Membership on an entity’s Board of Directors or advisory committees; JENSENN, Membership on an entity’s Board of Directors or advisory committees; GSK., Membership on an entity’s Board of Directors or advisory committees; JAZZ, Membership on an entity’s Board of Directors or advisory committees; GAS: AbbVie Inc, Allogene Therapeutics, Autolus Therapeutics, BeiGene Ltd, Bristol-Myers Squibb Company, Celgene Corporation, Debiopharm Group, Genmab, Kite, A Gilead Company, Incyte Corporation, Janssen Biotech Inc, Miltenyi Biotec, MorphoSys, Novartis, Roche, Advisory Committee; Bristol-Myers Squibb Company, Celgene Corporation, Debiopharm Group, Genmab, Kite, A Gilead Company, Incyte Corporation, Miltenyi Biotec, MorphoSys, Novartis, Roche Laboratories Inc, Consultancy; CSS: Juno Therapeutics, Consultancy and Research Funding; Sanofi-Genzyme, Consultancy and Research Funding; Spectrum Pharmaceuticals, Consultancy; Novartis, Consultancy; Genmab, Consultancy; Precision Biosciences, Consultancy; Kite/Gilead, Consultancy; Celgene, Consultancy and Research Funding; Gamida Cell, Consultancy; GSK., Consultancy; Bristol-Myers Squibb, Research Funding; MS: McKinsey & Company, Consultancy; Angiocrine Bioscience, Consultancy and Research Funding; Omeros Corporation, Consultancy and research funding; Amgen, Inc., Research funding; Kite - A Gilead Company, Advisory Board; i3 Health, Other: Honorarium, CME activity; Medscape, LLC, Other: Honorarium, CME activity; GS: Amgen, Research Funding; Janssen Pharmaceutica, Research Funding; AA: Takeda, Consultancy and honoraria; Janseen, Research Funding; BMS, Research Funding; Gilead, Consultancy and honoraria; Pfizer, Consultancy and honoraria; M-AP: Bristol-Myers Squibb, Honoraria; Celgene, Honoraria; Equilium, Honoraria; Incyte, Honoraria and Other: Clinical trial support to institution; Karyopharm, Honoraria; Kite/Gilead, Honoraria and Other: Clinical trial support to institution; Merck, Honoraria; Miltenyi Biotec, Honoraria and Other; MorphoSys, Honoraria; Novartis, Honoraria and Other: Clinical trial support to institution; Nektar Therapeutics, Honoraria and Other; Omeros, Honoraria; Takeda, Honoraria; Cidara Therapeutics, Honoraria; Medigene, Honoraria; Sellas Life Sciences, Honoraria; Servier, Honoraria; NexImmune, Honoraria; EJ: Novartis, Advisory board, Honoraria; JY: Convergent R.N.R Ltd., Advisory board; MR: Celgene, Provision of Services; Auron Therapeutics, Inc., Ownership/Equity Interests; Provision of Services; Physicians’ Education Resourc, Provision of Services. M.J.B. Envizion Medical, Membership on an entity’s Board of Directors or advisory committees; Biological Industries (a Sartorius Company), Gilboa Therapeutics, Consultancy; Envizion Medical, Other: Patents.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Previous Publication: This work was presented in part as an oral presentation at the 63rd American Society of Hematology Annual Meeting (Atlanta, GA).
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alarcon Tomas, A., Fein, J.A., Fried, S. et al. Outcomes of first therapy after CD19-CAR-T treatment failure in large B-cell lymphoma. Leukemia 37, 154–163 (2023). https://doi.org/10.1038/s41375-022-01739-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-022-01739-2