Abstract
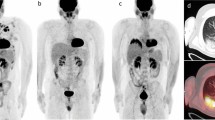
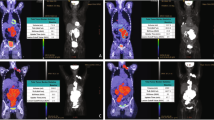
Risk-stratified treatment strategies have the potential to increase survival and lower toxicity in relapsed/refractory classical Hodgkin lymphoma (R/R cHL) patients. This study investigated the prognostic value of serum (s)TARC, vitamin D and lactate dehydrogenase (LDH), TARC immunohistochemistry and quantitative PET parameters in 65 R/R cHL patients who were treated with brentuximab vedotin (BV) and DHAP followed by autologous stem-cell transplantation (ASCT) within the Transplant BRaVE study (NCT02280993). At a median follow-up of 40 months, the 3-year progression free survival (PFS) was 77% (95% CI: 67–88%) and the overall survival was 95% (90–100%). Significant adverse prognostic markers for progression were weak/negative TARC staining of Hodgkin Reed-Sternberg cells in the baseline biopsy, and a high standard uptake value (SUV)mean or SUVpeak on the baseline PET scan. After one cycle of BV-DHAP, sTARC levels were strongly associated with the risk of progression using a cutoff of 500 pg/ml. On the pre-ASCT PET scan, SUVpeak was highly prognostic for progression post-ASCT. Vitamin D, LDH and metabolic tumor volume had low prognostic value. In conclusion, we established the prognostic impact of sTARC, TARC staining, and quantitative PET parameters for R/R cHL, allowing the use of these parameters in prospective risk-stratified clinical trials. Trial registration: NCT02280993.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
De-identified data will be shared with other researchers upon reasonable request to the corresponding authors (m.j.kersten@amsterdamumc.nl or a.diepstra@umcg.nl). The sharing will require a detailed proposal to the study investigators, and a data transfer agreement must be signed.
References
Schmitz N, Pfistner B, Sextro M, Sieber M, Carella AM, Haenel M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: a randomised trial. Lancet. 2002;359:2065–71.
Linch DC, Winfield D, Goldstone AH, Moir D, Hancock B, McMillan A, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin’s disease: results of a BNLI randomised trial. Lancet. 1993;341:1051–4.
Majhail NS, Weisdorf DJ, Defor TE, Miller JS, McGlave PB, Slungaard A, et al. Long-term results of autologous stem cell transplantation for primary refractory or relapsed Hodgkin’s lymphoma. Biol Blood Marrow Transpl. 2006;12:1065–72.
Josting A, Rudolph C, Mapara M, Glossmann JP, Sieniawski M, Sieber M, et al. Cologne high-dose sequential chemotherapy in relapsed and refractory Hodgkin lymphoma: results of a large multicenter study of the German Hodgkin Lymphoma Study Group (GHSG). Ann Oncol. 2005;16:116–23.
Moskowitz CH, Nimer SD, Zelenetz AD, Trippett T, Hedrick EE, Filippa DA, et al. A 2-step comprehensive high-dose chemoradiotherapy second-line program for relapsed and refractory Hodgkin disease: analysis by intent tot treat and development of a prognostic model. Blood. 2001;97:616–23.
Advani R, Moskowitz AJ, Bartlett NL, Vose J, Ramchandren R, Feldman T, et al. Brentuximab vedotin in combination with nivolumab in relapsed or refractory Hodgkin lymphoma: 3-year study results. Blood. 2021;138:427–38.
Moskowitz AJ, Shah G, Schöder H, Ganesan N, Drill E, Hancock H, et al. Phase II trial of pembrolizumab plus gemcitabine, vinorelbine, and liposomal doxorubicin as second-line therapy for relapsed or refractory classical Hodgkin lymphoma. J Clin Oncol. 2021;39:3109–17.
Kersten MJ, Driessen J, Zijlstra JM, Plattel WJ, Morschhauser F, Lugtenburg PJ, et al. Combining brentuximab vedotin with dexamethasone, high-dose cytarabine and cisplatin as salvage treatment in relapsed or refractory Hodgkin lymphoma: the phase II HOVON/LLPC Transplant BRaVE study. Haematologica. 2021;106:1129–37.
Moskowitz AJ, Schoder H, Gavane S, Thoren KL, Fleisher M, Yahalom J, et al. Prognostic significance of baseline metabolic tumor volume in relapsed and refractory Hodgkin lymphoma. Blood. 2017;130:2196–203.
Moskowitz CH, Nademanee A, Masszi T, Agura E, Holowiecki J, Abidi MH, et al. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;385:1853–62.
Herrera AF, Palmer J, Martin P, Armenian S, Tsai NC, Kennedy N, et al. Autologous stem-cell transplantation after second-line brentuximab vedotin in relapsed or refractory Hodgkin lymphoma. Ann Oncol. 2018;29:724–30.
Garcia-Sanz R, Sureda A, de la Cruz F, Canales M, Gonzalez AP, Pinana JL, et al. Brentuximab vedotin and ESHAP is highly effective as second-line therapy for Hodgkin lymphoma patients (long-term results of a trial by the Spanish GELTAMO Group). Ann Oncol. 2019;30:612–20.
LaCasce AS, Bociek RG, Sawas A, Caimi P, Agura E, Matous J, et al. Brentuximab vedotin plus bendamustine: a highly active first salvage regimen for relapsed or refractory Hodgkin lymphoma. Blood. 2018;132:40–8.
Broccoli A, Argnani L, Botto B, Corradini P, Pinto A, Re A, et al. First salvage treatment with bendamustine and brentuximab vedotin in Hodgkin lymphoma: a phase 2 study of the Fondazione Italiana Linfomi. Blood Cancer J. 2019;9:100.
Moskowitz CH, Matasar MJ, Zelenetz AD, Nimer SD, Gerecitano J, Hamlin P, et al. Normalization of pre-ASCT, FDG-PET imaging with second-line, non-cross-resistant, chemotherapy programs improves event-free survival in patients with Hodgkin lymphoma. Blood. 2012;119:1665–70.
Moskowitz CH, Yahalom J, Zelenetz AD, Zhang Z, Filippa D, Teruya-Feldstein J, et al. High-dose chemo-radiotherapy for relapsed or refractory Hodgkin lymphoma and the significance of pre-transplant functional imaging. Br J Haematol. 2010;148:890–7.
Devillier R, Coso D, Castagna L, Brenot Rossi I, Anastasia A, Chiti A, et al. Positron emission tomography response at the time of autologous stem cell transplantation predicts outcome of patients with relapsed and/or refractory Hodgkin’s lymphoma responding to prior salvage therapy. Haematologica. 2012;97:1073–9.
Burggraaff CN, Cornelisse AC, Hoekstra OS, Lugtenburg PJ, De Keizer B, Arens AIJ, et al. Interobserver agreement of interim and end-of-treatment (18)F-FDG PET/CT in diffuse large B-cell lymphoma: impact on clinical practice and trials. J Nucl Med. 2018;59:1831–6.
Cottereau AS, Versari A, Loft A, Casasnovas O, Bellei M, Ricci R, et al. Prognostic value of baseline metabolic tumor volume in early-stage Hodgkin lymphoma in the standard arm of the H10 trial. Blood. 2018;131:1456–63.
Song MK, Chung JS, Lee JJ, Jeong SY, Lee SM, Hong JS, et al. Metabolic tumor volume by positron emission tomography/computed tomography as a clinical parameter to determine therapeutic modality for early stage Hodgkin’s lymphoma. Cancer Sci. 2013;104:1656–61.
Procházka V, Gawande RS, Cayci Z, Froelich JW, Cao Q, Wilke C, et al. Positron emission tomography-based assessment of metabolic tumor volume predicts survival after autologous hematopoietic cell transplantation for Hodgkin lymphoma. Biol Blood Marrow Transpl. 2018;24:64–70.
Plattel WJ, van den Berg A, Visser L, van der Graaf AM, Pruim J, Vos H, et al. Plasma thymus and activation-regulated chemokine as an early response marker in classical Hodgkin’s lymphoma. Haematologica. 2012;97:410–5.
Plattel WJ, Visser L, Diepstra A, Glaudemans A, Nijland M, van Meerten T, et al. Interim thymus and activation regulated chemokine versus interim (18) F-fluorodeoxyglucose positron-emission tomography in classical Hodgkin lymphoma response evaluation. Br J Haematol. 2020;190:40–4.
van den Berg A, Visser L, Poppema S. High expression of the CC chemokine TARC in Reed-sternberg cells. Am J Pathol. 1999;154:1685–91.
Plattel WJ, Alsada ZN, van Imhoff GW, Diepstra A, van den Berg A, Visser L. Biomarkers for evaluation of treatment response in classical Hodgkin lymphoma: comparison of sGalectin-1, sCD163 and sCD30 with TARC. Br J Haematol. 2016;175:868–75.
Qin JQ, Yin H, Wu JZ, Chen RZ, Xia Y, Wang L, et al. 25-Hydroxy vitamin D deficiency predicts inferior prognosis in Hodgkin lymphoma. Leuk Res. 2021;105:106580.
Borchmann S, Cirillo M, Goergen H, Meder L, Sasse S, Kreissl S, et al. Pretreatment vitamin D deficiency is associated with impaired progression-free and overall survival in Hodgkin lymphoma. J Clin Oncol. 2019;37:3528–37.
Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–54.
Kaalep A, Sera T, Oyen W, Krause BJ, Chiti A, Liu Y, et al. EANM/EARL FDG-PET/CT accreditation—summary results from the first 200 accredited imaging systems. Eur J Nucl Med Mol Imaging. 2018;45:412–22.
Boellaard R. Quantitative oncology molecular analysis suite: ACCURATE. J Nucl Med. 2018;59:1753.
Driessen J, Zwezerijnen GJ, Schöder H, Drees EE, Kersten MJ, Moskowitz AJ, et al. The impact of semi-automatic segmentation methods on metabolic tumor volume, intensity and dissemination radiomics in (18)F-FDG PET scans of patients with classical Hodgkin lymphoma. J Nucl Med. 2022;63:1424–30.
Eertink JJ, van de Brug T, Wiegers SE, Zwezerijnen GJC, Pfaehler EAG, Lugtenburg PJ, et al. 18F-FDG PET baseline radiomics features improve the prediction of treatment outcome in diffuse large B-cell lymphoma. Eur J Nucl Med Mol Imaging. 2021;49:932–43.
Barrington SF, Kluge R. FDG PET for therapy monitoring in Hodgkin and non-Hodgkin lymphomas. Eur J Nucl Med Mol Imaging. 2017;44:97–110.
Boktor RR, Walker G, Stacey R, Gledhill S, Pitman AG. Reference range for intrapatient variability in blood-pool and liver SUV for 18F-FDG PET. J Nucl Med. 2013;54:677–82.
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–68.
OpenClinica. LLC and collaborators; 2004–2018.
Josting A, Muller H, Borchmann P, Baars JW, Metzner B, Dohner H, et al. Dose intensity of chemotherapy in patients with relapsed Hodgkin’s lymphoma. J Clin Oncol. 2010;28:5074–80.
von Tresckow B, Muller H, Eichenauer DA, Glossmann JP, Josting A, Boll B, et al. Outcome and risk factors of patients with Hodgkin Lymphoma who relapse or progress after autologous stem cell transplant. Leuk Lymphoma. 2014;55:1922–4.
Santoro A, Magagnoli M, Spina F, Pinotti G, Siracusano L, Michieli M, et al. Ifosfamide, gemcitabine, and vinorelbine: a new induction regimen for refractory and relapsed Hodgkin’s lymphoma. Haematologica. 2007;92:35–41.
Chen R, Zinzani PL, Fanale MA, Armand P, Johnson NA, Brice P, et al. Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J Clin Oncol. 2017;35:2125–32.
Armand P, Chen YB, Redd RA, Joyce RM, Bsat J, Jeter E, et al. PD-1 blockade with pembrolizumab for classical Hodgkin lymphoma after autologous stem cell transplantation. Blood. 2019;134:22–9.
Herrera AF, Chen L, Nieto Y, Holmberg L, Johnston PB, Mei M, et al. Consolidation with nivolumab and brentuximab Vedotin after autologous hematopoietic cell transplantation in patients with high-risk Hodgkin lymphoma. Blood. 2020;136:19–20.
Farina L, Rezzonico F, Spina F, Dodero A, Mazzocchi A, Crippa F, et al. Serum thymus and activation-regulated chemokine level monitoring may predict disease relapse detected by PET scan after reduced-intensity allogeneic stem cell transplantation in patients with Hodgkin lymphoma. Biol Blood Marrow Transpl. 2014;20:1982–8.
Sobesky S, Mammadova L, Cirillo M, Drees EEE, Mattlener J, Dörr H, et al. In-depth cell-free DNA sequencing reveals genomic landscape of Hodgkin’s lymphoma and facilitates ultrasensitive residual disease detection. Med. 2021;2:1171–93.e11.
Ma Y, Visser L, Roelofsen H, de Vries M, Diepstra A, van Imhoff G, et al. Proteomics analysis of Hodgkin lymphoma: identification of new players involved in the cross-talk between HRS cells and infiltrating lymphocytes. Blood. 2008;111:2339–46.
Acknowledgements
We would like to dedicate this article to the memory of Professor Anton Hagenbeek, who together with MJK initiated this study. The authors would like to thank all patients who participated in the trial, the Transplant BRaVE-trial team of the Trial Office of the Amsterdam UMC, location AMC for their efforts in trial management and central data management, and the members of the Data Safety and Monitoring Board. The authors thank Marjolein Spiering, Edith van Dijkman, the data managers, trial nurses, lab- and pharmacy personnel for their essential assistance with collecting and managing the study data. The authors thank Nathalie Hijmering, HOVON Pathology Facility and Biobank, for biopsy collection and support of central pathology review.
Funding
This work was supported by research funding from Takeda.
Author information
Authors and Affiliations
Consortia
Contributions
MJK and Anton Hagenbeek designed and supervised the clinical study. AD supervised the biomarker study. All authors collected the data. JD and LV performed biomarker analysis. JD performed the PET segmentation. GJCZ and RB supervised the PET segmentation. DdJ and AD performed the central pathology review. AA, RV and GJCZ performed the central PET-CT review. JD performed the statistical analysis. JD wrote the manuscript with contributions from all authors. All authors interpreted the data, read, commented on, and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The study drug (BV) was provided for the study and the study was funded by Takeda. Takeda did not have any influence on the data analysis or the interpretation of the results. MJK: honoraria from and consulting/advisory role for BMS/Celgene, Kite, a Gilead Company, Miltenyi Biotech, Novartis, Adicet Bio and Roche; research funding from Kite, a Gilead Company, and Takeda; and travel support from Kite, A Gilead Company, Miltenyi Biotech, Novartis, and Roche. MH: Consultant/advisor: Roche, Takeda, Celgene, Genmab; Research support: Roche, Takeda, Celgene, Genmab, Novartis, Janssen, Incyte, Genentech. PJL: honoraria from and consulting/advisory role for Takeda, Servier, Roche, Genmab, AbbVie, Incyte, Regeneron, Celgene; Research funding from Takeda, Servier and Roche. FM: advisory boards for Roche, BMS, Genmab, Abbvie, Miltenyi, Novartis, Gilead, Asrtra Zeneca. Scientific lectures for Roche, Janssen. Consultancy for Roche, Genmab, Abbvie, Gilead. AD: Millennium/Takeda: Consultancy, Honoraria, Research Funding. TG: Millennium/Takeda: Honoraria, Gilead, Roche, MSD. JMZ: Consultant/advisor: Gilead, Roche, Takeda; Honoraria: Gilead, Roche, Takeda, Janssen. DdJ: Consultant/advisor: Takeda. SHT: Consultant/advisor: Takeda, Kite/Gilead; Research Funding: Beigene. The other authors declare no potential conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Driessen, J., Kersten, M.J., Visser, L. et al. Prognostic value of TARC and quantitative PET parameters in relapsed or refractory Hodgkin lymphoma patients treated with brentuximab vedotin and DHAP. Leukemia 36, 2853–2862 (2022). https://doi.org/10.1038/s41375-022-01717-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-022-01717-8
This article is cited by
-
Quantitative PET-based biomarkers in lymphoma: getting ready for primetime
Nature Reviews Clinical Oncology (2023)