Abstract
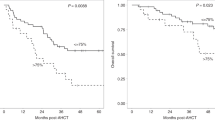
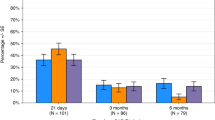
Conditioning chemotherapy (CCT) has been shown to be essential for optimal efficacy of chimeric antigen receptor (CAR) T-cell therapy. Here, we determined whether the change in absolute lymphocyte count, referred to as delta lymphocyte index (DLIx), may serve as a surrogate marker for pharmacodynamic effects of CCT and whether it associated with germline genetic variants in patients with large B-cell lymphoma (LBCL). One-hundred and seventy-one patients were included, of which 86 (50%) received bridging therapy post-leukapheresis. Median DLIx was 0.5 × 109/L (range, 0.01–2.75 × 109/L) and was significantly higher in patients who achieved complete response (p = 0.04). On multivariate analysis, low DLIx was associated only with use of bridging therapy (odds ratio 0.4, 95% CI 0.2–0.8, p = 0.007). Low DLIx was independently associated with shorter progression-free (p = 0.02) and overall survival (p = 0.02). DLIx was associated with genetic variations related to drug metabolism and macrophage biology such as ABCB1, MISP and CPVL. The impact of CCT on lymphocyte count is affected by use of bridging therapy but change in lymphocyte count is independently associated with efficacy. Studies aimed at investigating macrophage biology in this setting may suggest strategies to increase the efficacy of CCT and improve outcomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Muranski P, Boni A, Wrzesinski C, Citrin DE, Rosenberg SA, Childs R, et al. Increased intensity lymphodepletion and adoptive immunotherapy-how far can we go? Nat Clin Pr Oncol. 2006;3:668–81.
Neelapu SS. CAR-T efficacy: is conditioning the key? Blood. 2019;133:1799–1800.
Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202:907–12.
Kochenderfer JN, Somerville RPT, Lu T, Shi V, Bot A, Rossi J, et al. Lymphoma remissions caused by anti-CD19 chimeric antigen receptor T cells are associated with high serum Interleukin-15 levels. J Clin Oncol. 2017;35:1803–13.
Turtle CJ, Hanafi LA, Berger C, Gooley TA, Cherian S, Hudecek M, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126:2123–38.
Hirayama AV, Gauthier J, Hay KA, Voutsinas JM, Wu Q, Gooley T, et al. The response to lymphodepletion impacts PFS in patients with aggressive non-Hodgkin lymphoma treated with CD19 CAR T cells. Blood. 2019;133:1876–87.
Andreadis C, Tam CS, Borchmann P, Jaeger U, McGuirk JP, Holte H, et al. Correlation of bridging and lymphodepleting chemotherapy with clinical outcomes in patients with relapsed/refractory diffuse large B-Cell lymphoma treated with Tisagenlecleucel. Blood. 2019;134:2883–2883.
Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FL, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15:47–62.
Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transpl. 2019;25:625–38.
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55.
Sehn LH, Berry B, Chhanabhai M, Fitzgerald C, Gill K, Hoskins P, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007;109:1857–61.
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–68.
Das S, Forer L, Schonherr S, Sidore C, Locke AE, Kwong A, et al. Next-generation genotype imputation service and methods. Nat Genet. 2016;48:1284–7.
Whirl-Carrillo M, Huddart R, Gong L, Sangkuhl K, Thorn CF, Whaley R, et al. An evidence-based framework for evaluating pharmacogenomics knowledge for personalized medicine. Clin Pharm Ther. 2021;110:563–72.
Wu MC, Lee S, Cai T, Li Y, Boehnke M, Lin X. Rare-variant association testing for sequencing data with the sequence kernel association test. Am J Hum Genet. 2011;89:82–93.
Nastoupil LJ, Jain MD, Feng L, Spiegel JY, Ghobadi A, Lin Y, et al. Standard-of-Care Axicabtagene Ciloleucel for relapsed or refractory large B-cell lymphoma: results from the US Lymphoma CAR T Consortium. J Clin Oncol. 2020;38:3119–28.
Jacobson CA, Hunter BD, Redd R, Rodig SJ, Chen PH, Wright K, et al. Axicabtagene Ciloleucel in the non-trial setting: outcomes and correlates of response, resistance, and toxicity. J Clin Oncol. 2020;38:3095–106.
Dwivedy Nasta S, Hughes ME, Namoglu EC, Landsburg DJ, Chong EA, Barta SK, et al. A characterization of bridging therapies leading up to commercial CAR T-Cell therapy. Blood. 2019;134:4108–4108.
Paillassa J, Vercellino L, Di Blasi R, Bernard S, Moatti H, Bommier C, et al. Impact of bridging chemotherapy on clinical outcomes of CD19 CAR T therapy in relapse/refractory diffuse large B- cell lymphoma in real world experience. Blood. 2019;134:2886–2886.
Sim AJ, Jain MD, Figura NB, Chavez JC, Shah BD, Khimani F, et al. Radiation Therapy as a bridging strategy for CAR T cell therapy with axicabtagene ciloleucel in diffuse large B-Cell Lymphoma. Int J Radiat Oncol Biol Phys. 2019;105:1012–21.
Imber B, Palomba ML, DeSelm C, Batlevi CL, Dahi PB, Giralt SA, et al. MSKCC Early experience using radiotherapy as a bridging strategy for relapsed diffuse large B Cell Lymphoma before CD19 CAR T Therapy. Blood. 2019;134:3238–3238.
Pinnix CC, Gunther JR, Dabaja BS, Strati P, Fang P, Hawkins MC, et al. Bridging therapy prior to axicabtagene ciloleucel for relapsed/refractory large B-cell lymphoma. Blood Adv. 2020;4:2871–83.
Bertholee D, Maring JG, van Kuilenburg AB. Genotypes affecting the pharmacokinetics of anticancer drugs. Clin Pharmacokinet. 2017;56:317–37.
Dimeloe S, Frick C, Fischer M, Gubser PM, Razik L, Bantug GR, et al. Human regulatory T cells lack the cyclophosphamide-extruding transporter ABCB1 and are more susceptible to cyclophosphamide-induced apoptosis. Eur J Immunol. 2014;44:3614–20.
Rivero A, Rapado I, Tomas JF, Montalban C, de Ona R, Paz-Carreira J, et al. Relationship between deoxycytidine kinase (DCK) genotypic variants and fludarabine toxicity in patients with follicular lymphoma. Leuk Res. 2011;35:431–7.
Johnson GG, Lin K, Cox TF, Oates M, Sibson DR, Eccles R, et al. CYP2B6*6 is an independent determinant of inferior response to fludarabine plus cyclophosphamide in chronic lymphocytic leukemia. Blood. 2013;122:4253–8.
Ekhart C, Rodenhuis S, Smits PH, Beijnen JH, Huitema AD. Relations between polymorphisms in drug-metabolising enzymes and toxicity of chemotherapy with cyclophosphamide, thiotepa and carboplatin. Pharmacogenet Genomics. 2008;18:1009–15.
Maier B, Kirsch M, Anderhub S, Zentgraf H, Kramer A. The novel actin/focal adhesion-associated protein MISP is involved in mitotic spindle positioning in human cells. Cell Cycle. 2013;12:1457–71.
Kumeta M, Gilmore JL, Umeshima H, Ishikawa M, Kitajiri S, Horigome T, et al. Caprice/MISP is a novel F-actin bundling protein critical for actin-based cytoskeletal reorganizations. Genes Cells. 2014;19:338–49.
Mahoney JA, Ntolosi B, DaSilva RP, Gordon S, McKnight AJ. Cloning and characterization of CPVL, a novel serine carboxypeptidase, from human macrophages. Genomics. 2001;72:243–51.
Harris J, Schwinn N, Mahoney JA, Lin HH, Shaw M, Howard CJ, et al. A vitellogenic-like carboxypeptidase expressed by human macrophages is localized in endoplasmic reticulum and membrane ruffles. Int J Exp Pathol. 2006;87:29–39.
Jain MD, Zhao H, Wang X, Atkins R, Menges M, Reid K, et al. Tumor interferon signaling and suppressive myeloid cells are associated with CAR T-cell failure in large B-cell lymphoma. Blood. 2021;137:2621–33.
Strati P, Neelapu SS. CAR-T failure: beyond antigen loss and T cells. Blood. 2021;137:2567–8.
Ansell SM, Flinn IW, Maris MB, O’Connor OA, Lesokhin A, Advani AS, et al. TTI-621 (SIRPaFc), an immune checkpoint inhibitor blocking the CD47 “Do Not Eat” Signal, induces objective responses in patients with advanced, relapsed/refractory diffuse large B-Cell Lymphoma (DLBCL). Blood 2017; 130.
Advani RHFI, Popplewell L, Forero-Torres A, Bartlett NL, Ghosh N, Kline JP, et al. Activity and tolerabilty of the first-in-class anti-CD47 antibody Hu5F9-G4 with rituximab tolerated in relapsed/refractory non-Hodgkin lymphoma: Initial phase 1b/2 results. J Clin Oncol. 2018; 36 (suppl; abstr 7504).
Lakhani NJLP, Hafez N, Krishnamurthy A, O’Rourke TJ, Kamdar MK, Fanning P, et al. A phase 1 study of ALX148, a CD47 blocker, alone and in combination with established anticancer antibodies in patients with advanced malignancy and non-Hodgkin lymphoma. J Clin Oncol. 2018; 36 (suppl; abstr 3068).
Kim TM, Lakhani N, Gainor J, Kamdar M, Fanning P, Squifflet P, et al. A Phase 1 Study of ALX148, a CD47 Blocker, in Combination with Rituximab in Patients with Non-Hodgkin Lymphoma. Blood. 2019;134:1953–1953.
Advani R, Flinn I, Popplewell L, Forero A, Bartlett NL, Ghosh N, et al. CD47 Blockade by Hu5F9-G4 and Rituximab in Non-Hodgkin’s Lymphoma. N. Engl J Med. 2018;379:1711–21.
Acknowledgements
This research is supported in part by the University of Texas MD Anderson Cancer Center B-cell Lymphoma Moonshot (SSN) and The University of Texas MD Anderson Cancer Center Support Grant from National Institutes of Health (P30 CA016672) and by the Shirley Stein Scientific Endowment Research Award. PS salary is supported by the Lymphoma Research Foundation Career Development Award, the Leukemia Lymphoma Society Career Development Program, the Kite Gilead Scholar in Clinical Research Award, the Sabin Fellowship Award, and by the R21 NIH grant. The MD Anderson Lymphoma Tissue Bank is supported by the KW Cares Foundation.
Author information
Authors and Affiliations
Contributions
APJ analyzed data, and wrote the paper; SA, HJL, SPY, RN, LJN, RES, EJS, PK, CRF, and JRW provided clinical care to patients and coauthored the paper; HM, BP and MN collected clinical data and coauthored the paper; KD, HJC, and NS performed cytokine analysis and co-authored the paper; CDH, YY, and MATH performed genetic analysis and coauthored the paper; RS and JC provided statistical support and coauthored the paper; PS and SSN designed the study, analyzed the data, provided clinical care to patients, and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
PS is a consultant or has served on advisory boards for Kite Gilead, Sobi, Roche-Genentech, Hutchinson MediPharma, ADC Therapeutics, Incyte Morphosys, and TG Therapeutics, and received research funds from Sobi, Astrazeneca-Acerta, ADC Therapeutics, and ALX Oncology. SSN has received personal fees from Kite, a Gilead Company, Merck, Bristol Myers Squibb, Novartis, Celgene, Pfizer, Allogene Therapeutics, Sellas Life Sciences, Cell Medica/Kuur/Athenex, Incyte, Precision Biosciences, Legend Biotech, Adicet Bio, Calibr, Unum Therapeutics, Bluebird Bio, and Sana Biotechnology; research support from Kite, a Gilead Company, Bristol Myers Squibb, Merck, Poseida, Cellectis, Celgene, Karus Therapeutics, Unum Therapeutics, Allogene Therapeutics, Precision Biosciences, Acerta, and Adicet Bio; royalties from Takeda Pharmaceuticals; and has intellectual property related to cell therapy.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Strati, P., Jallouk, A.P., Sun, R. et al. Impact of conditioning chemotherapy on lymphocyte kinetics and outcomes in LBCL patients treated with CAR T-cell therapy. Leukemia 36, 2669–2677 (2022). https://doi.org/10.1038/s41375-022-01704-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-022-01704-z
This article is cited by
-
“Digital twins elucidate critical role of Tscm in clinical persistence of TCR-engineered cell therapy”
npj Systems Biology and Applications (2024)