Abstract
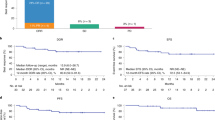
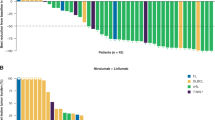
Richter’s Syndrome (RS) is an aggressive transformation of CLL, usually clonally-related diffuse large B-cell lymphoma (DLBCL), characterized by frequent TP53 mutations, intrinsic chemoresistance and poor survival. TP53-independent treatments are needed. We conducted a single center, phase 2, investigator-initiated study of high dose blinatumomab (maximum 112 mcg/d after initial, weekly dose escalation), NCT03121534, given for an 8-week induction and 4-week consolidation cycle. Responses were assessed by Lugano 2014 criteria. Serial multi-parameter flow cytometry from blood was performed to identify patient-specific biomarkers for response. Nine patients were treated. Patients had received a median of 4 and 2 prior therapies for CLL and RS, respectively. Five of 9 had del(17p) and 100% had complex karyotype. Four patients had reduction in nodal disease, including one durable complete response lasting >1 y. Treatment was well tolerated, with no grade >3 cytokine release syndrome and 1 case of grade 3, reversible neurotoxicity. Immunophenotyping demonstrated the majority of patients expressed multiple immune checkpoints, especially PD1, TIM3 and TIGIT. The patient who achieved CR had the lowest levels of immune checkpoint expression. Simultaneous targeting with immune checkpoint blockade, especially PD1 inhibition, which has already demonstrated single-agent efficacy in RS, could achieve synergistic killing and enhance outcomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Deidentified data will be shared with other researchers upon reasonable request to the corresponding author (pathompson2@mdanderson.org). The sharing will require a detailed proposal to the study investigators, and a data transfer agreement must be signed.
References
Rossi D, Spina V, Gaidano G. Biology and treatment of Richter syndrome. Blood. 2018;131:2761–72.
Younes A, Brody J, Carpio C, Lopez-Guillermo A, Ben-Yehuda D, Ferhanoglu B, et al. Safety and activity of ibrutinib in combination with nivolumab in patients with relapsed non-Hodgkin lymphoma or chronic lymphocytic leukaemia: a phase 1/2a study. Lancet Haematol. 2019;6:e67–78.
Gökbuget N, Dombret H, Bonifacio M, Reichle A, Graux C, Faul C, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. 2018;131:1522–31.
Topp MS, Gokbuget N, Stein AS, Zugmaier G, O’Brien S, Bargou RC, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16:57–66.
Viardot A, Goebeler M-E, Hess G, Neumann S, Pfreundschuh M, Adrian N, et al. Phase 2 study of bispecific T-cell engager (BiTE®) antibody blinatumomab in relapsed/refractory diffuse large B cell lymphoma. Blood. 2016;127:1410–6.
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–68.
Thall PF, Sung HG. Some extensions and applications of a Bayesian strategy for monitoring multiple outcomes in clinical trials. Stat Med. 1998;17:1563–80.
Fraietta JA, Lacey SF, Orlando EJ, Pruteanu-Malinici I, Gohil M, Lundh S, et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med. 2018;24:563–71.
Shah NN, Fry TJ. Mechanisms of resistance to CAR T cell therapy. Nature reviews. Clin Oncol. 2019;16:372–85.
Hutchings M, Morschhauser F, Iacoboni G, Carlo-Stella C, Offner FC, Sureda A, et al. Glofitamab, a Novel, Bivalent CD20-Targeting T-Cell–Engaging Bispecific Antibody, Induces Durable Complete Remissions in Relapsed or Refractory B-Cell Lymphoma: A Phase I Trial. J Clin Oncol. 2021;39:1959–70.
Hutchings M, Mous R, Clausen MR, Johnson P, Linton KM, Chamuleau MED, et al. Dose escalation of subcutaneous epcoritamab in patients with relapsed or refractory B-cell non-Hodgkin lymphoma: an open-label, phase 1/2 study. Lancet 2021;398:1157–69.
Ying Z, Huang XF, Xiang X, Liu Y, Kang X, Song Y, et al. A safe and potent anti-CD19 CAR T cell therapy. Nat Med. 2019;25:947–53.
Kittai AS, Bond DA, William B, Saad A, Penza S, Efebera Y, et al. Clinical activity of axicabtagene ciloleucel in adult patients with Richter syndrome. Blood Adv. 2020;4:4648–52.
Ding W, LaPlant BR, Call TG, Parikh SA, Leis JF, He R, et al. Pembrolizumab in patients with CLL and Richter transformation or with relapsed CLL. Blood 2017;129:3419.
Acknowledgements
This was supported, in part, by M.D. Anderson Cancer Center Support Grant P30 CA016672. The study drug and funding for the study was provided by Amgen. Amgen had no role in the conduct or analysis of the study or the writing of the paper.
Author information
Authors and Affiliations
Contributions
PAT designed and wrote the protocol, provided clinical care to patients, analyzed results and wrote the paper; XJ performed bioinformatic analysis of flow cytometry data and wrote the paper; KC developed the strategic plan for the computational analysis team; PB and KR designed and performed experiments and co-wrote the paper; RB performed experiments and assisted in data analysis; MK, VN and AKNC performed experiments; AF, MK and MA provided clinical care to patients; CBP provided the statistical design for the protocol; NG centrally analyzed PETCT results; WGW assisted in protocol design and planning, provided clinical care to patients and co-wrote the paper. All authors provided intellectual input, critically evaluated the final draft and approved it for submission.
Corresponding author
Ethics declarations
Competing interests
PAT consulted for Amgen, Genentech and AbbVie. The remainder of the authors declare no relevant conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Thompson, P.A., Jiang, X., Banerjee, P. et al. A phase two study of high dose blinatumomab in Richter’s syndrome. Leukemia 36, 2228–2232 (2022). https://doi.org/10.1038/s41375-022-01649-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-022-01649-3
This article is cited by
-
Richter Transformation of Chronic Lymphocytic Leukemia—Are We Making Progress?
Current Hematologic Malignancy Reports (2023)