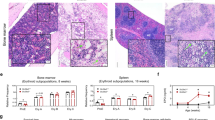
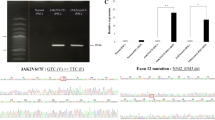
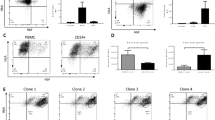
Abstract
Although a glycosylphosphatidylinositol-anchored protein (GPI-AP) CD109 serves as a TGF-β co-receptor and inhibits TGF-β signaling in keratinocytes, the role of CD109 on hematopoietic stem progenitor cells (HSPCs) remains unknown. We studied the effect of CD109 knockout (KO) or knockdown (KD) on TF-1, a myeloid leukemia cell line that expresses CD109, and primary human HSPCs. CD109-KO or KD TF-1 cells underwent erythroid differentiation in the presence of TGF-β. CD109 was more abundantly expressed in hematopoietic stem cells (HSCs) than in multipotent progenitors and HSPCs of human bone marrow (BM) and cord blood but was not detected in mouse HSCs. Erythroid differentiation was induced by TGF-β to a greater extent in CD109-KD cord blood or iPS cell-derived megakaryocyte–erythrocyte progenitor cells (MEPs) than in wild-type MEPs. When we analyzed the phenotype of peripheral blood MEPs of patients with paroxysmal nocturnal hemoglobinuria who had both GPI(+) and GPI(−) CD34+ cells, the CD36 expression was more evident in CD109− MEPs than CD109+ MEPs. In summary, CD109 suppresses TGF-β signaling in HSPCs, and the lack of CD109 may increase the sensitivity of PIGA-mutated HSPCs to TGF-β, thus leading to the preferential commitment of erythroid progenitor cells to mature red blood cells in immune-mediated BM failure.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Tutelman PR, Aubert G, Milner RA, Dalal BI, Schultz KR, Deyell RJ. Paroxysmal nocturnal haemoglobinuria phenotype cells and leucocyte subset telomere length in childhood acquired aplastic anaemia. Br J Haematol. 2014;164:717–21.
Kulagin A, Lisukov I, Ivanova M, Golubovskaya I, Kruchkova I, Bondarenko S, et al. Prognostic value of paroxysmal nocturnal haemoglobinuria clone presence in aplastic anaemia patients treated with combined immunosuppression: results of two-centre prospective study. Br J Haematol. 2014;164:546–54.
Sugimori C, Chuhjo T, Feng X, Yamazaki H, Takami A, Teramura M, et al. Minor population of CD55-CD59- blood cells predicts response to immunosuppressive therapy and prognosis in patients with aplastic anemia. Blood. 2006;107:1308–14.
Gargiulo L, Papaioannou M, Sica M, Talini G, Chaidos A, Richichi B, et al. Glycosylphosphatidylinositol-specific, CD1d-restricted T cells in paroxysmal nocturnal hemoglobinuria. Blood. 2013;121:2753–61.
Hosokawa K, Katagiri T, Sugimori N, Ishiyama K, Sasaki Y, Seiki Y, et al. Favorable outcome of patients who have 13q deletion: a suggestion for revision of the WHO ‘MDS-U’ designation. Haematologica. 2012;97:1845–9.
Zhang H, Kozono DE, O’Connor KW, Vidal-Cardenas S, Rousseau A, Hamilton A, et al. TGF-beta inhibition rescues hematopoietic stem cell defects and bone marrow failure in fanconi anemia. Cell Stem Cell. 2016;18:668–81.
de Bruin AM, Voermans C, Nolte MA. Impact of interferon-gamma on hematopoiesis. Blood. 2014;124:2479–86.
Massague J. TGFbeta signalling in context. Nat Rev Mol Cell Biol. 2012;13:616–30.
Garbe A, Spyridonidis A, Mobest D, Schmoor C, Mertelsmann R, Henschler R. Transforming growth factor-beta 1 delays formation of granulocyte-macrophage colony-forming cells, but spares more primitive progenitors during ex vivo expansion of CD34+ haemopoietic progenitor cells. Br J Haematol. 1997;99:951–8.
Batard P, Monier MN, Fortunel N, Ducos K, Sansilvestri-Morel P, Phan T, et al. TGF-(beta)1 maintains hematopoietic immaturity by a reversible negative control of cell cycle and induces CD34 antigen up-modulation. J Cell Sci. 2000;113:383–90.
Sitnicka E, Ruscetti FW, Priestley GV, Wolf NS, Bartelmez SH. Transforming growth factor beta 1 directly and reversibly inhibits the initial cell divisions of long-term repopulating hematopoietic stem cells. Blood. 1996;88:82–8.
Fortunel NO, Hatzfeld A, Hatzfeld JA. Transforming growth factor-beta: pleiotropic role in the regulation of hematopoiesis. Blood. 2000;96:2022–36.
He W, Dorn DC, Erdjument-Bromage H, Tempst P, Moore MA, Massague J. Hematopoiesis controlled by distinct TIF1gamma and Smad4 branches of the TGFbeta pathway. Cell. 2006;125:929–41.
Finnson KW, Tam BY, Liu K, Marcoux A, Lepage P, Roy S, et al. Identification of CD109 as part of the TGF-beta receptor system in human keratinocytes. FASEB J. 2006;20:1525–7.
Murray LJ, Bruno E, Uchida N, Hoffman R, Nayar R, Yeo EL, et al. CD109 is expressed on a subpopulation of CD34+ cells enriched in hematopoietic stem and progenitor cells. Exp Hematol. 1999;27:1282–94.
Zhang JM, Murakumo Y, Hagiwara S, Jiang P, Mii S, Kalyoncu E, et al. CD109 attenuates TGF-beta1 signaling and enhances EGF signaling in SK-MG-1 human glioblastoma cells. Biochem Biophys Res Commun. 2015;459:252–8.
Mii S, Murakumo Y, Asai N, Jijiwa M, Hagiwara S, Kato T, et al. Epidermal hyperplasia and appendage abnormalities in mice lacking CD109. Am J Pathol. 2012;181:1180–9.
Chuang CH, Greenside PG, Rogers ZN, Brady JJ, Yang D, Ma RK, et al. Molecular definition of a metastatic lung cancer state reveals a targetable CD109-Janus kinase-Stat axis. Nat Med. 2017;23:291–300.
Litvinov IV, Bizet AA, Binamer Y, Jones DA, Sasseville D, Philip A. CD109 release from the cell surface in human keratinocytes regulates TGF-beta receptor expression, TGF-beta signalling and STAT3 activation: relevance to psoriasis. Exp Dermatol. 2011;20:627–32.
Hagiwara S, Murakumo Y, Mii S, Shigetomi T, Yamamoto N, Furue H, et al. Processing of CD109 by furin and its role in the regulation of TGF-beta signaling. Oncogene. 2010;29:2181–91.
Xue Q, Yu C, Wang Y, Liu L, Zhang K, Fang C, et al. miR-9 and miR-124 synergistically affect regulation of dendritic branching via the AKT/GSK3beta pathway by targeting Rap2a. Sci Rep. 2016;6:26781.
Kitamura T, Tange T, Terasawa T, Chiba S, Kuwaki T, Miyagawa K, et al. Establishment and characterization of a unique human cell line that proliferates dependently on GM-CSF, IL-3, or erythropoietin. J Cell Physiol. 1989;140:323–34.
Klampfer L, Zhang J, Nimer SD. GM-CSF rescues TF-1 cells from growth factor withdrawal-induced, but not differentiation-induced apoptosis: the role of BCL-2 and MCL-1. Cytokine. 1999;11:849–55.
Mori Y, Chen JY, Pluvinage JV, Seita J, Weissman IL. Prospective isolation of human erythroid lineage-committed progenitors. Proc Natl Acad Sci USA. 2015;112:9638–43.
Manz MG, Miyamoto T, Akashi K, Weissman IL. Prospective isolation of human clonogenic common myeloid progenitors. Proc Natl Acad Sci USA. 2002;99:11872–7.
Majeti R, Park CY, Weissman IL. Identification of a hierarchy of multipotent hematopoietic progenitors in human cord blood. Cell Stem Cell. 2007;1:635–45.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Oida T, Weiner HL. Depletion of TGF-beta from fetal bovine serum. J Immunol Methods. 2010;362:195–8.
Espinoza JL, Elbadry MI, Chonabayashi K, Yoshida Y, Katagiri T, Harada K, et al. Hematopoiesis by iPSC-derived hematopoietic stem cells of aplastic anemia that escape cytotoxic T-cell attack. Blood Adv. 2018;2:390–400.
Liu L, Liu X, Ren X, Tian Y, Chen Z, Xu X, et al. Smad2 and Smad3 have differential sensitivity in relaying TGFbeta signaling and inversely regulate early lineage specification. Sci Rep. 2016;6:21602.
Frick CL, Yarka C, Nunns H, Goentoro L. Sensing relative signal in the Tgf-beta/Smad pathway. Proc Natl Acad Sci USA. 2017;114:E2975–82.
Ohshima Y, Yajima I, Kumasaka MY, Yanagishita T, Watanabe D, Takahashi M, et al. CD109 expression levels in malignant melanoma. J Dermatol Sci. 2010;57:140–2.
Bizet AA, Liu K, Tran-Khanh N, Saksena A, Vorstenbosch J, Finnson KW, et al. The TGF-beta co-receptor, CD109, promotes internalization and degradation of TGF-beta receptors. Biochim Biophys Acta. 2011;1813:742–53.
Vorstenbosch J, Al-Ajmi H, Winocour S, Trzeciak A, Lessard L, Philip A. CD109 overexpression ameliorates skin fibrosis in a mouse model of bleomycin-induced scleroderma. Arthritis Rheum. 2013;65:1378–83.
Zhou S, da Silva SD, Siegel PM, Philip A. CD109 acts as a gatekeeper of the epithelial trait by suppressing epithelial to mesenchymal transition in squamous cell carcinoma cells in vitro. Sci Rep. 2019;9:16317.
Yamazaki S, Iwama A, Takayanagi S, Eto K, Ema H, Nakauchi H. TGF-beta as a candidate bone marrow niche signal to induce hematopoietic stem cell hibernation. Blood. 2009;113:1250–6.
Langer JC, Henckaerts E, Orenstein J, Snoeck HW. Quantitative trait analysis reveals transforming growth factor-beta2 as a positive regulator of early hematopoietic progenitor and stem cell function. J Exp Med. 2004;199:5–14.
Hosokawa K, Mizumaki H, Elbadry MI, Saito C, Espinoza JL, Thi Thanh Dao A, et al. Clonal hematopoiesis by SLIT1-mutated hematopoietic stem cells due to a breakdown of the autocrine loop involving Slit1 in acquired aplastic anemia. Leukemia. 2019;33:2732–66.
Geutskens SB, Andrews WD, van Stalborch AM, Brussen K, Holtrop-de Haan SE, Parnavelas JG, et al. Control of human hematopoietic stem/progenitor cell migration by the extracellular matrix protein Slit3. Lab Investig. 2012;92:1129–39.
Shibata F, Goto-Koshino Y, Morikawa Y, Komori T, Ito M, Fukuchi Y, et al. Roundabout 4 is expressed on hematopoietic stem cells and potentially involved in the niche-mediated regulation of the side population phenotype. Stem Cells. 2009;27:183–90.
Khurana S, Margamuljana L, Joseph C, Schouteden S, Buckley SM, Verfaillie CM. Glypican-3-mediated inhibition of CD26 by TFPI: a novel mechanism in hematopoietic stem cell homing and maintenance. Blood. 2013;121:2587–95.
Mine T, Harada K, Matsumoto T, Yamana H, Shirouzu K, Itoh K, et al. CDw108 expression during T-cell development. Tissue Antigens. 2000;55:429–36.
Acknowledgements
This work was supported by MEXT KAKENHI (Grant-in-Aid for Scientific Research (B), Grant Number: 24390243) to SN, MEXT KAKENHI (Grant-in-Aid for Young Scientists (B), Grant Number: 26860363) to TK, MEXT KAKENHI (Grant-in-Aid for Young Scientists, Grant Number: 17K16184 and 19K17823) to KH, MEXT KAKENHI (Grant-in-Aid for Scientific Research (C), Grant Number: 18K08318) to HY. We are grateful to the following doctors providing us with technical advice: Prof. Toshio Kitamura (The institute of Medial Sciences, The University of Tokyo, Japan) and Shinji Mii (Nagoya University). We thank the patients and donors and their physicians for contributing to this study and the Advanced Preventive Medical Sciences Research Center, Kanazawa University for the use of facilities. MT is a Ph.D. candidate at Kanazawa University, and this work is submitted in partial fulfillment of the requirements for the Ph.D.
Author information
Authors and Affiliations
Contributions
MT, KH, NN, NT, RU, HY, and SN collected clinical data and blood and BM samples. MT, KH, MATN, NN, LE, MIE, and MM, performed most of the in vitro experiments. KM, TK, and AH, performed in vivo experiments. MO and HF collected cord blood samples. KC and YY generated induced pluripotent stem cells. MT, KH, and SN designed the research and wrote the paper. All authors critically reviewed the paper and checked the final version. MT, KH, and MATN contributed equally to this work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Tanabe, M., Hosokawa, K., Nguyen, M.A.T. et al. The GPI-anchored protein CD109 protects hematopoietic progenitor cells from undergoing erythroid differentiation induced by TGF-β. Leukemia 36, 847–855 (2022). https://doi.org/10.1038/s41375-021-01463-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-021-01463-3