Abstract
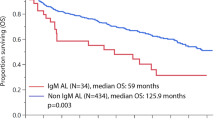
The association between familial plasma cell disorders (PCD) and prognosis in patients with MGUS, multiple myeloma (MM), and systemic light chain (AL) amyloidosis has not been well described. This study retrospectively reviewed outcomes of 25,423 patients (16,744 MGUS, 6194 MM, 2955 AL amyloidosis). Overall, 2.7% of patients reported having a family member with a PCD (defined as MGUS, MM, or AL amyloidosis). Family history was documented in 94% of MGUS, 92% of MM, and 88% of AL amyloidosis patients. The overall survival was consistently longer in patients with versus without familial PCD (crude hazard ratios: 0.52, 95% CI 0.40–0.67, p < 0.001 for MGUS patients; 0.68, 95% CI 0.57–0.79, p < 0.001 for MM patients; 0.60, 95% CI 0.43–0.84, p = 0.003 for AL patients). This association remained consistent when adjusting for baseline patient and disease characteristics. In MGUS patients, the risk of progression to MM, AL amyloidosis, or a lymphoproliferative disorder was higher in patients with familial PCD when accounting for death as a competing risk (cause-specific HR 1.9, 95% 1.3–2.7, p < 0.001). This is the first study to demonstrate that in a cohort of MGUS, MM, and systemic AL amyloidosis, patients with a PCD family history have an improved overall survival.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, Offord JR, et al. Prevalence of monoclonal gammopathy of undetermined significance. N. Engl J Med. 2006;354:1362–9.
Kyle RA, Larson DR, Therneau TM, Dispenzieri A, Kumar S, Cerhan JR, et al. Long-term follow-up of monoclonal gammopathy of undetermined significance. N. Engl J Med. 2018;378:241–9.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30.
Quock TP, Yan T, Chang E, Guthrie S, Broder MS. Epidemiology of AL amyloidosis: a real-world study using US claims data. Blood Adv. 2018;2:1046–53.
Mandema E, Wildervanck LS. La maladie de Kahler (myelomes multiples) chez deuz soeurs. J Genet Hum. 1954;3:170–5.
Jain M, Ascensao J, Schechter GP. Familial myeloma and monoclonal gammopathy: a report of eight African American families. Am J Hematol. 2009;84:34–8.
Grosbois B, Gueguen M, Fauchet R, Lebouc H, Guenot A, Lancelin F, et al. Multiple myeloma in two brothers. An immunochemical and immunogenetic familial study. Cancer. 1986;58:2417–21.
Grosbois B, Jego P, Attal M, Payen C, Rapp MJ, Fuzibet JG, et al. Familial multiple myeloma: report of fifteen families. Br J Haematol. 1999;105:768–70.
Bizzaro N, Pasini P. Familial occurrence of multiple myeloma and monoclonal gammopathy of undetermined significance in 5 siblings. Haematologica. 1990;75:58–63.
Lynch HT, Sanger WG, Pirruccello S, Quinn-Laquer B, Weisenburger DD. Familial multiple myeloma: a family study and review of the literature. J Natl Cancer Inst. 2001;93:1479–83.
Lynch HT, Ferrara K, Barlogie B, Coleman EA, Lynch JF, Weisenburger D, et al. Familial myeloma. N. Engl J Med. 2008;359:152–7.
Morgan GJ, Johnson DC, Weinhold N, Goldschmidt H, Landgren O, Lynch HT, et al. Inherited genetic susceptibility to multiple myeloma. Leukemia. 2014;28:518–24.
Landgren O, Linet MS, McMaster ML, Gridley G, Hemminki K, Goldin LR. Familial characteristics of autoimmune and hematologic disorders in 8,406 multiple myeloma patients: a population-based case-control study. Int J Cancer. 2006;118:3095–8.
Landgren O, Kristinsson SY, Goldin LR, Caporaso NE, Blimark C, Mellqvist UH, et al. Risk of plasma cell and lymphoproliferative disorders among 14621 first-degree relatives of 4458 patients with monoclonal gammopathy of undetermined significance in Sweden. Blood. 2009;114:791–5.
Kristinsson SY, Bjorkholm M, Goldin LR, Blimark C, Mellqvist UH, Wahlin A, et al. Patterns of hematologic malignancies and solid tumors among 37,838 first-degree relatives of 13,896 patients with multiple myeloma in Sweden. Int J Cancer. 2009;125:2147–50.
Vachon CM, Kyle RA, Therneau TM, Foreman BJ, Larson DR, Colby CL, et al. Increased risk of monoclonal gammopathy in first-degree relatives of patients with multiple myeloma or monoclonal gammopathy of undetermined significance. Blood. 2009;114:785–90.
Benson MD, Liepnieks JJ, Kluve-Beckerman B. Hereditary systemic immunoglobulin light-chain amyloidosis. Blood. 2015;125:3281–6.
Pertesi M, Went M, Hansson M, Hemminki K, Houlston RS, Nilsson B. Genetic predisposition for multiple myeloma. Leukemia. 2020;34:697–708.
Went M, Sud A, Försti A, Halvarsson B-M, Weinhold N, Kimber S, et al. Identification of multiple risk loci and regulatory mechanisms influencing susceptibility to multiple myeloma. Nature Commun. 2018;9:1–8.
Greenberg AJ, Cousin M, Kumar S, Ketterling RP, Knudson RA, Larson D, et al. Differences in the distribution of cytogenetic subtypes between multiple myeloma patients with and without a family history of monoclonal gammopathy and multiple myeloma. Eur J Haematol. 2013;91:193–5.
Greenberg AJ, Rajkumar SV, Larson DR, Dispenzieri A, Therneau TM, Colby CL, et al. Increased prevalence of light chain monoclonal gammopathy of undetermined significance (LC-MGUS) in first-degree relatives of individuals with multiple myeloma. Br J Haematol. 2012;157:472–5.
Clay-Gilmour AI, Kumar S, Rajkumar SV, Rishi A, Kyle RA, Katzmann JA, et al. Risk of MGUS in relatives of multiple myeloma cases by clinical and tumor characteristics. Leukemia. 2019;33:499–507.
Fermand J-P, Bridoux F, Dispenzieri A, Jaccard A, Kyle RA, Leung N, et al. Monoclonal gammopathy of clinical significance: a novel concept with therapeutic implications. Blood. 2018;132:1478–85.
Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–48.
Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc. 1999;94:496.
Aradóttir K, Lund S, Landgren O, Bjorkholm M, Turesson I, Kristinsson S. Family history of lymphoproliferative disease associated with a suprior survival in multiple myeloma: a popoulation-based study (Abstract P292). Eur Hematol Association. 2015. https://library.ehaweb.org/eha/2015/20th/100541/kristrn.aradttir.family.history.of.lymphoproliferative.disease.associated.with.html.
Wei X, Calvo-Vidal MN, Chen S, Wu G, Revuelta MV, Sun J, et al. Germline Lysine-Specific Demethylase 1 (LSD1/KDM1A) Mutations Confer Susceptibility to Multiple Myeloma. Cancer Res. 2018;78:2747–59.
Dilworth D, Liu L, Stewart AK, Berenson JR, Lassam N, Hogg D. Germline CDKN2A mutation implicated in predisposition to multiple myeloma. Blood. 2000;95:1869–71.
Catalano C, Paramasivam N, Blocka J, Giangiobbe S, Huhn S, Schlesner M, et al. Characterization of rare germline variants in familial multiple myeloma. Blood Cancer J. 2021;11:33.
Lonial S, Jacobus S, Fonseca R, Weiss M, Kumar S, Orlowski RZ, et al. Randomized trial of lenalidomide versus observation in smoldering multiple myeloma. J Clin Oncol. 2020;38:1126–37.
Mateos M-V, Hernández M-T, Giraldo P, De La Rubia J, De Arriba F, Corral LL, et al. Lenalidomide plus dexamethasone for high-risk smoldering multiple myeloma. N. Engl J Med. 2013;369:438–47.
Fiederling J, Shams AZ, Haug U. Validity of self-reported family history of cancer: a systematic literature review on selected cancers. Int J Cancer. 2016;139:1449–60.
Chang ET, Smedby KE, Hjalgrim H, Glimelius B, Adami H-O. Reliability of self-reported family history of cancer in a large case–control study of lymphoma. JNCI: J Natl Cancer Inst. 2006;98:61–8.
Landgren O, Katzmann JA, Hsing AW, Pfeiffer RM, Kyle RA, Yeboah ED, et al. Prevalence of monoclonal gammopathy of undetermined significance among men in Ghana. Mayo Clin Proc. 2007;82:1468–73.
Landgren O, Gridley G, Turesson I, Caporaso NE, Goldin LR, Baris D, et al. Risk of monoclonal gammopathy of undetermined significance (MGUS) and subsequent multiple myeloma among African American and white veterans in the United States. Blood 2006;107:904–6.
Brown LM, Linet MS, Greenberg RS, Silverman DT, Hayes RB, Swanson GM, et al. Multiple myeloma and family history of cancer among blacks and whites in the U.S. Cancer. 1999;85:2385–90.
Author information
Authors and Affiliations
Contributions
AV, CV, and SK contributed to the study design, data collection and analysis, and drafting of the manuscript. DL provided statistical support. LB, JS, AD, PK, MQL, MAG, FKB, SRH, DD, TK, WG, RW, EM, NL, RAK, SVR, SK contributed to manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Visram, A., Vachon, C., Baughn, L.B. et al. Family history of plasma cell disorders is associated with improved survival in MGUS, multiple myeloma, and systemic AL amyloidosis. Leukemia 36, 1058–1065 (2022). https://doi.org/10.1038/s41375-021-01454-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-021-01454-4