Abstract
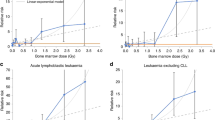
There is limited evidence that non-leukaemic lymphoid malignancies are radiogenic. As radiation-related cancer risks are generally higher after childhood exposure, we analysed pooled lymphoid neoplasm data in nine cohorts first exposed to external radiation aged <21 years using active bone marrow (ABM) and, where available, lymphoid system doses, and harmonised outcome classification. Relative and absolute risk models were fitted. Years of entry spanned 1916–1981. At the end of follow-up (mean 42.1 years) there were 593 lymphoma (422 non-Hodgkin (NHL), 107 Hodgkin (HL), 64 uncertain subtype), 66 chronic lymphocytic leukaemia (CLL) and 122 multiple myeloma (MM) deaths and incident cases among 143,136 persons, with mean ABM dose 0.14 Gy (range 0–5.95 Gy) and mean age at first exposure 6.93 years. Excess relative risk (ERR) was not significantly increased for lymphoma (ERR/Gy = −0.001; 95% CI: −0.255, 0.279), HL (ERR/Gy = −0.113; 95% CI: −0.669, 0.709), NHL + CLL (ERR/Gy = 0.099; 95% CI: −0.149, 0.433), NHL (ERR/Gy = 0.068; 95% CI: −0.253, 0.421), CLL (ERR/Gy = 0.320; 95% CI: −0.678, 1.712), or MM (ERR/Gy = 0.149; 95% CI: −0.513, 1.063) (all p-trend > 0.4). In six cohorts with estimates of lymphatic tissue dose, borderline significant increased risks (p-trend = 0.02–0.07) were observed for NHL + CLL, NHL, and CLL. Further pooled epidemiological studies are needed with longer follow-up, central outcome review by expert hematopathologists, and assessment of radiation doses to lymphoid tissues.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The data is available from the principal author upon request.
Code availability
All analysis code is available from the principal author upon request.
Change history
26 October 2021
The name tagging of author Amy Berrington de Gonzalez was updated.
References
United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). UNSCEAR 2006 Report. Annex A. Epidemiological studies of radiation and cancer. New York: United Nations; 2008. p. 13–322.
Linet MS, Schubauer-Berigan MK, Weisenburger DD, Richardson DB, Landgren O, Blair A, et al. Chronic lymphocytic leukaemia: an overview of aetiology in light of recent developments in classification and pathogenesis. Br J Haematol. 2007;139:672–86.
Pierce DA, Shimizu Y, Preston DL, Vaeth M, Mabuchi K. Studies of the mortality of atomic bomb survivors. Report 12, Part I. Cancer: 1950-1990. Radiat Res. 1996;146:1–27.
Armstrong B, Brenner DJ, Baverstock K, Cardis E, Green A, Guilmette RA, et al. Radiation. Volume 100D. A review of human carcinogens. Lyon, France: International Agency for Research on Cancer; 2012. p. 1–341.
Richardson DB, Sugiyama H, Wing S, Sakata R, Grant E, Shimizu Y, et al. Positive associations between ionizing radiation and lymphoma mortality among men. Am J Epidemiol. 2009;169:969–76.
Hsu W-L, Preston DL, Soda M, Sugiyama H, Funamoto S, Kodama K, et al. The incidence of leukemia, lymphoma and multiple myeloma among atomic bomb survivors: 1950-2001. Radiat Res. 2013;179:361–82.
Kim CJ, Freedman DM, Curtis RE, Berrington de Gonzalez A, Morton LM. Risk of non-Hodgkin lymphoma after radiotherapy for solid cancers. Leuk Lymphoma. 2013;54:1691–7.
Leuraud K, Richardson DB, Cardis E, Daniels RD, Gillies M, O’Hagan JA, et al. Ionising radiation and risk of death from leukaemia and lymphoma in radiation-monitored workers (INWORKS): an international cohort study. Lancet Haematol. 2015;2:e276–e281.
Zablotska LB, Bazyka D, Lubin JH, Gudzenko N, Little MP, Hatch M, et al. Radiation and the risk of chronic lymphocytic and other leukemias among Chornobyl cleanup workers. Environ Health Perspect. 2013;121:59–65.
Harbron RW, Chapple CL, O’Sullivan JJ, Lee C, McHugh K, Higueras M, et al. Cancer incidence among children and young adults who have undergone x-ray guided cardiac catheterization procedures. Eur J Epidemiol. 2018;33:393–401.
Harbron RW, Pasqual E. Ionising radiation as a risk factor for lymphoma: a review. J Radio Prot. 2020;40:R151–R185.
Hunter N, Haylock R. Radiation risks of lymphoma and multiple myeloma incidence in the updated NRRW-3 cohort in the UK: 1950-2011. J Radiol Prot. 2021. https://doi.org/10.1088/1361-6498/abee96.
Kesminiene A, Evrard AS, Ivanov VK, Malakhova IV, Kurtinaitis J, Stengrevics A, et al. Risk of hematological malignancies among Chernobyl liquidators. Radiat Res. 2008;170:721–35.
Little MP, Wakeford R, Borrego D, French B, Zablotska LB, Adams MJ, et al. Leukaemia and myeloid malignancy among people exposed to low doses (<100 mSv) of ionising radiation during childhood: a pooled analysis of nine historical cohort studies. Lancet Haematol. 2018;5:e346–e358.
Candéias SM, Kabacik S, Olsen A-K, Eide DM, Brede DA, Bouffler S, et al. Ionizing radiation does not impair the mechanisms controlling genetic stability during T cell receptor gene rearrangement in mice. Int J Radiat Biol. 2018;94:357–65.
Johnsen HE, Bogsted M, Schmitz A, Bodker JS, El-Galaly TC, Johansen P, et al. The myeloma stem cell concept, revisited: from phenomenology to operational terms. Haematologica. 2016;101:1451–9.
International Commission on Radiological Protection (ICRP). Stem cell biology with respect to carcinogenesis aspects of radiological protection. ICRP Publication 131. Ann ICRP. 2015;44(3–4):1–357.
Gault N, Verbiest T, Badie C, Romeo P-H, Bouffler S. Haematopoietic stem and progenitor cell responses to low radiation doses-implications for leukaemia risk. Int J Radiat Biol. 2019:95:892–9.
Lee C, Morton LM, Berrington de Gonzalez A. A novel method to estimate lymphocyte dose and application to pediatric and young adult CT patients in the United Kingdom. Radiat Prot Dosim. 2018;178:116–21.
Little MP. Leukaemia following childhood radiation exposure in the Japanese atomic bomb survivors and in medically exposed groups. Radiat Prot Dosim. 2008;132:156–65.
Krishnan B, Morgan GJ. Non-Hodgkin lymphoma secondary to cancer chemotherapy. Cancer Epidemiol Biomark Prev. 2007;16:377–80.
Davis FG, Boice JD Jr., Hrubec Z, Monson RR. Cancer mortality in a radiation-exposed cohort of Massachusetts tuberculosis patients. Cancer Res. 1989;49:6130–6.
Zablotska LB, Little MP, Cornett RJ. Potential increased risk of ischemic heart disease mortality with significant dose fractionation in the Canadian fluoroscopy cohort study. Am J Epidemiol. 2014;179:120–31.
Dondon MG, de Vathaire F, Shamsaldin A, Doyon F, Diallo I, Ligot L, et al. Cancer mortality after radiotherapy for a skin hemangioma during childhood. Radiother Oncol. 2004;72:87–93.
Lindberg S, Karlsson P, Arvidsson B, Holmberg E, Lunberg LM, Wallgren A. Cancer incidence after radiotherapy for skin haemangioma during infancy. Acta Oncol. 1995;34:735–40.
Lundell M, Holm L-E. Mortality from leukemia after irradiation in infancy for skin hemangioma. Radiat Res. 1996;145:595–601.
Lundell M, Mattsson A, Karlsson P, Holmberg E, Gustafsson A, Holm L-E. Breast cancer risk after radiotherapy in infancy: a pooled analysis of two Swedish cohorts of 17,202 infants. Radiat Res. 1999;151:626–32.
Sadetzki S, Chetrit A, Lubina A, Stovall M, Novikov I. Risk of thyroid cancer after childhood exposure to ionizing radiation for tinea capitis. J Clin Endocrinol Metab. 2006;91:4798–804.
Ron E, Modan B, Boice JD Jr. Mortality after radiotherapy for ringworm of the scalp. Am J Epidemiol. 1988;127:713–25.
Adams MJ, Dozier A, Shore RE, Lipshultz SE, Schwartz RG, Constine LS, et al. Breast cancer risk 55+ years after irradiation for an enlarged thymus and its implications for early childhood medical irradiation today. Cancer Epidemiol Biomark Prev. 2010;19:48–58.
Adams MJ, Shore RE, Dozier A, Lipshultz SE, Schwartz RG, Constine LS, et al. Thyroid cancer risk 40+ years after irradiation for an enlarged thymus: an update of the Hempelmann cohort. Radiat Res. 2010;174:753–62.
Ronckers CM, Land CE, Miller JS, Stovall M, Lonstein JE, Doody MM. Cancer mortality among women frequently exposed to radiographic examinations for spinal disorders. Radiat Res. 2010;174:83–90.
Lee C, Lamart S, Moroz BE. Computational lymphatic node models in pediatric and adult hybrid phantoms for radiation dosimetry. Phys Med Biol. 2013;58:N59–82.
Sadetzki S, Chetrit A, Mandelzweig L, Nahon D, Freedman L, Susser E, et al. Childhood exposure to ionizing radiation to the head and risk of schizophrenia. Radiat Res. 2011;176:670–7.
Jaffe ES, Harris NL, Stein H, Isaacson PG. Classification of lymphoid neoplasms: the microscope as a tool for disease discovery. Blood. 2008;112:4384–99.
Morton LM, Turner JJ, Cerhan JR, Linet MS, Treseler PA, Clarke CA, et al. Proposed classification of lymphoid neoplasms for epidemiologic research from the Pathology Working Group of the International Lymphoma Epidemiology Consortium (InterLymph). Blood. 2007;110:695–708.
Turner JJ, Morton LM, Linet MS, Clarke CA, Kadin ME, Vajdic CM, et al. InterLymph hierarchical classification of lymphoid neoplasms for epidemiologic research based on the WHO classification (2008): update and future directions. Blood. 2010;116:e90–98.
Yu E-M, Kittai A, Tabbara IA. Chronic lymphocytic leukemia: current concepts. Anticancer Res. 2015;35:5149–65.
Jaffe ES, Harris NL, Stein H, Vardiman JW. World Health Organization classification of tumours. Pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press; 2001. p. 1–352.
Kendall GM, Little MP, Wakeford R, Bunch KJ, Miles JCH, Vincent TJ, et al. A record-based case-control study of natural background radiation and the incidence of childhood leukaemia and other cancers in Great Britain during 1980-2006. Leukemia. 2013;27:3–9.
Spycher BD, Lupatsch JE, Zwahlen M, Roosli M, Niggli F, Grotzer MA, et al. Background ionizing radiation and the risk of childhood cancer: a census-based nationwide cohort study. Environ Health Perspect. 2015;123:622–8.
Krestinina LY, Davis FG, Schonfeld S, Preston DL, Degteva M, Epifanova S, et al. Leukaemia incidence in the Techa River Cohort: 1953-2007. Br J Cancer. 2013;109:2886–93.
Hastie TJ, Tibshirani RJ. Generalized additive models. Boca Raton, FL: Chapman & Hall/CRC; 1990. p. 1–350.
McCullagh P, Nelder JA. Generalized linear models. 2nd ed. Boca Raton, FL: Chapman and Hall/CRC; 1989. p. 1–526.
Risk Sciences International. Epicure version 2.0.1.0. 55 Metcalfe, K1P 6L5. Canada: Risk Sciences International;; 2015.
Little MP, Wakeford R, Lubin JH, Kendall GM. The statistical power of epidemiological studies analyzing the relationship between exposure to ionizing radiation and cancer, with special reference to childhood leukemia and natural background radiation. Radiat Res. 2010;174:387–402.
International Commission on Radiological Protection (ICRP). Basic anatomical and physiological data for use in radiological protection: reference values. ICRP Publication 89. Ann ICRP. 2001;32(3–4):1–265. i-xi.
International Commission on Radiological Protection (ICRP). Report of the Task Group on reference man. ICRP Publication 23. Ann ICRP. 1975;23:1–480.
Linet MS, Schubauer-Berigan MK, Berrington de Gonzalez A. Outcome assessment in epidemiological studies of low-dose radiation exposure and cancer risks: sources, level of ascertainment, and misclassification. J Natl Cancer Inst Monogr. 2020;2020:154–75.
Little MP, Weiss HA, Boice JD Jr., Darby SC, Day NE, Muirhead CR. Risks of leukemia in Japanese atomic bomb survivors, in women treated for cervical cancer, and in patients treated for ankylosing spondylitis. Radiat Res. 1999;152:280–92.
Richardson DB, Wing S, Schroeder J, Schmitz-Feuerhake I, Hoffmann W. Ionizing radiation and chronic lymphocytic leukemia. Environ Health Perspect. 2005;113:1–5.
Schubauer-Berigan MK, Daniels RD, Fleming DA, Markey AM, Couch JR, Ahrenholz SH, et al. Chronic lymphocytic leukaemia and radiation: findings among workers at five US nuclear facilities and a review of the recent literature. Br J Haematol. 2007;139:799–808.
Zent CS, Kyasa MJ, Evans R, Schichman SA. Chronic lymphocytic leukemia incidence is substantially higher than estimated from tumor registry data. Cancer. 2001;92:1325–30.
Turesson I, Linet MS, Bjorkholm M, Kristinsson SY, Goldin LR, Caporaso NE, et al. Ascertainment and diagnostic accuracy for hematopoietic lymphoproliferative malignancies in Sweden 1964-2003. Int J Cancer. 2007;121:2260–6.
Acknowledgements
This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics. The Radiation Effects Research Foundation (RERF), Hiroshima and Nagasaki, Japan is a public interest foundation funded by the Japanese Ministry of Health, Labour and Welfare (MHLW) and the US Department of Energy (DOE). The research was also funded in part through DOE award DE-HS0000031 to the National Academy of Sciences. Dr. Zablotska’s work was supported by National Cancer Institute of the National Institutes of Health under awards R03CA188614 and R01CA197422. This publication was supported by RERF Research Protocol A1-16. The views of the authors do not necessarily reflect those of the two governments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
RW receives a consultancy fee as a member of the Technical Working Party of the Compensation Scheme for Radiation-linked Diseases (http://www.csrld.org.uk). No other authors report conflicts of interest.
Ethical approval
The study cohort has been declared exempt by the National Cancer Institute Special Studies Institution Review Board, because using pre-existing approved data. Obtaining informed consent from all study subjects was therefore not necessary.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Little, M.P., Wakeford, R., Zablotska, L.B. et al. Lymphoma and multiple myeloma in cohorts of persons exposed to ionising radiation at a young age. Leukemia 35, 2906–2916 (2021). https://doi.org/10.1038/s41375-021-01284-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-021-01284-4
This article is cited by
-
Radiation exposure and leukaemia risk among cohorts of persons exposed to low and moderate doses of external ionising radiation in childhood
British Journal of Cancer (2023)
-
Lympho-hematopoietic malignancies risk after exposure to low dose ionizing radiation during cardiac catheterization in childhood
European Journal of Epidemiology (2023)
-
Childhood cancer risks estimates following CT scans: an update of the French CT cohort study
European Radiology (2022)