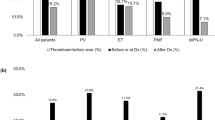
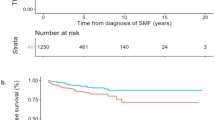
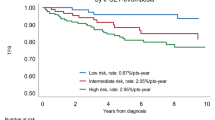
Abstract
Thrombosis, both in arterial and venous territories, is the major complication of myeloproliferative neoplasms and is responsible for a high rate of morbidity and mortality. The currently accepted risk factors are an age over 60 years and a history of thrombosis. However, many complex mechanisms contribute to this increased prothrombotic risk, with involvement of all blood cell types, plasmatic factors, and endothelial cells. Besides, some cardiovascular events may originate from arterial vasospasm that could contribute to thrombotic complications. In this review, we discuss recent results obtained in mouse models in the light of data obtained from clinical studies. We emphasize on actors of thrombosis that are currently not targeted with current therapeutics but could be promising targets, i.e, neutrophil extracellular traps and vascular reactivity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–405.
Rungjirajittranon T, Owattanapanich W, Ungprasert P, Siritanaratkul N, Ruchutrakool T. A systematic review and meta-analysis of the prevalence of thrombosis and bleeding at diagnosis of Philadelphia-negative myeloproliferative neoplasms. BMC Cancer. 2019;19:184.
Marchioli R, Finazzi G, Landolfi R, Kutti J, Gisslinger H, Patrono C, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol. 2005;23:2224–32.
Sekhar M, McVinnie K, Burroughs AK. Splanchnic vein thrombosis in myeloproliferative neoplasms. Br J Haematol. 2013;162:730–47.
Marchioli R, Finazzi G, Specchia G, Cacciola R, Cavazzina R, Cilloni D, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013;368:22–33.
Carobbio A, Thiele J, Passamonti F, Rumi E, Ruggeri M, Rodeghiero F, et al. Risk factors for arterial and venous thrombosis in WHO-defined essential thrombocythemia: an international study of 891 patients. Blood. 2011;117:5857–9.
Barbui T, Finazzi G, Carobbio A, Thiele J, Passamonti F, Rumi E, et al. Development and validation of an International Prognostic Score of thrombosis in World Health Organization-essential thrombocythemia (IPSET-thrombosis). Blood. 2012;120:5128–33.
Tefferi A, Barbui T. Polycythemia vera and essential thrombocythemia: 2021 update on diagnosis, risk-stratification and management. Am J Hematol. 2020;95:1599–613. https://onlinelibrary.wiley.com/doi/abs/10.1002/ajh.26008
Maslah N, Soret J, Dosquet C, Vercellino L, Belkhodja C, Schlageter M-H, et al. Masked polycythemia vera: analysis of a single center cohort of 2480 red cell masses. Haematologica. 2020;105:e95–7.
Hultcrantz M, Björkholm M, Dickman PW, Landgren O, Derolf ÅR, Kristinsson SY, et al. Risk for arterial and venous thrombosis in patients with myeloproliferative neoplasms: a population-based cohort study. Ann Intern Med. 2018;168:317.
De Stefano V, Ruggeri M, Cervantes F, Alvarez-Larrán A, Iurlo A, Randi ML, et al. High rate of recurrent venous thromboembolism in patients with myeloproliferative neoplasms and effect of prophylaxis with vitamin K antagonists. Leukemia. 2016;30:2032–8.
Stein BL, Martin K. From Budd-Chiari syndrome to acquired von Willebrand syndrome: thrombosis and bleeding complications in the myeloproliferative neoplasms. Hematology Am Soc Hematol Educ Program. 2019;2019:397–406.
Weisel JW, Litvinov RI. Red blood cells: the forgotten player in hemostasis and thrombosis. J Thromb Haemost. 2019;17:271–82.
Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss D, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–5.
Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci. 2010;107:15880–5.
von Brühl M-L, Stark K, Steinhart A, Chandraratne S, Konrad I, Lorenz M, et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012;209:819–35.
Massberg S, Grahl L, von Bruehl M-L, Manukyan D, Pfeiler S, Goosmann C, et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med. 2010;16:887–96.
Campbell PJ, MacLean C, Beer PA, Buck G, Wheatley K, Kiladjian J-J, et al. Correlation of blood counts with vascular complications in essential thrombocythemia: analysis of the prospective PT1 cohort. Blood. 2012;120:1409–11.
Jensen MK, Brown PDN, Lund BV, Nielsen OJ, Hasselbalch HC. Increased platelet activation and abnormal membrane glycoprotein content and redistribution in myeloproliferative disorders. Br J Haematol. 2000;110:116–24.
Falanga A, Marchetti M, Vignoli A, Balducci D, Barbui T. Leukocyte-platelet interaction in patients with essential thrombocythemia and polycythemia vera. Exp Hematol. 2005;33:523–30.
Arellano-Rodrigo E, Alvarez-Larrán A, Reverter JC, Villamor N, Colomer D, Cervantes F. Increased platelet and leukocyte activation as contributing mechanisms for thrombosis in essential thrombocythemia and correlation with the JAK2 mutational status. Haematologica. 2006;169:75.
Falanga A, Marchetti M, Vignoli A, Balducci D, Russo L, Guerini V, et al. V617F JAK-2 mutation in patients with essential thrombocythemia: relation to platelet, granulocyte, and plasma hemostatic and inflammatory molecules. Exp Hematol. 2007;35:702–11.
Arellano‐Rodrigo E, Alvarez‐Larrán A, Reverter J-C, Colomer D, Villamor N, Bellosillo B, et al. Platelet turnover, coagulation factors, and soluble markers of platelet and endothelial activation in essential thrombocythemia: Relationship with thrombosis occurrence and JAK2 V617F allele burden. Am J Hematol. 2008;84:102–8.
Panova-Noeva M, Marchetti M, Buoro S, Russo L, Leuzzi A, Finazzi G, et al. JAK2V617F mutation and hydroxyurea treatment as determinants of immature platelet parameters in essential thrombocythemia and polycythemia vera patients. Blood. 2011;118:2599–601.
Landolfi R, Ciabattoni G, Patrignani P, Bizzi B, Patrono C. Increased thromboxane biosynthesis in patients with polycythemia vera: evidence for aspirin-suppressible platelet activation in vivo. Blood. 1992;8:1965–71.
Pareti FI, Gugliotta L, Mannucci L, Guarini A, Mannucci PM. Biochemical and metabolic aspects of platelet dysfunction in chronic myeloproliferative disorders. Thromb Haemost. 1982;47:84–9.
Landolfi R, Rocca B, Patrono C. Bleeding and thrombosis in myeloproliferative disorders: mechanisms and treatment. Crit Rev Oncol Hematol. 1995;20:203–22.
Schafer AI. Bleeding and thrombosis in the myeloproliferative disorders. Blood. 1984;64:1–12.
Panova‐Noeva M, Marchetti M, Spronk HM, Russo L, Diani E, Finazzi G, et al. Platelet-induced thrombin generation by the calibrated automated thrombogram assay is increased in patients with essential thrombocythemia and polycythemia vera. Am J Hematol. 2011;86:337–42.
Tiedt R, Schomber T, Hao-Shen H, Skoda RC. Pf4-Cre transgenic mice allow the generation of lineage-restricted gene knockouts for studying megakaryocyte and platelet function in vivo. Blood. 2007;109:1503–6.
Mansier O, Kilani B, Guitart AV, Guy A, Gourdou-Latyszenok V, Marty C, et al. Description of a knock-in mouse model of JAK2V617F MPN emerging from a minority of mutated hematopoietic stem cells. Blood. 2019;134:2383–7.
Calaminus SDJ, Guitart A, Sinclair A, Schachtner H, Watson SP, Holyoake TL, et al. Lineage tracing of Pf4-Cre marks hematopoietic stem cells and their progeny. PLoS One. 2012;7:e51361.
Lamrani L, Lacout C, Ollivier V, Denis CV, Gardiner E, Ho Tin Noe B, et al. Hemostatic disorders in a JAK2V617F-driven mouse model of myeloproliferative neoplasm. Blood. 2014;124:1136–45.
Etheridge SL, Roh ME, Cosgrove ME, Sangkhae V, Fox NE, Chen J, et al. JAK2V617F-positive endothelial cells contribute to clotting abnormalities in myeloproliferative neoplasms. Proc Natl Acad Sci. 2014;111:2295–300.
Strassel C, Kubovcakova L, Mangin PH, Ravanat C, Freund M, Skoda RC, et al. Haemorrhagic and thrombotic diatheses in mouse models with thrombocytosis. Thromb Haemost. 2015;113:414–25.
Hobbs CM, Manning H, Bennett C, Vasquez L, Severin S, Brain L, et al. JAK2V617F leads to intrinsic changes in platelet formation and reactivity in a knock-in mouse model of essential thrombocythemia. Blood. 2013;122:3787–97.
Pearson T. Hemorheologic considerations in the pathogenesis of vascular occlusive events in polycythemia vera. Semin Thromb Hemost. 1997;23:433–9.
Zhao B, Keerthivasan G, Mei Y, Yang J, McElherne J, Wong P, et al. Targeted shRNA screening identified critical roles of pleckstrin-2 in erythropoiesis. Haematologica. 2014;99:1157–67.
Zhao B, Mei Y, Cao L, Zhang J, Sumagin R, Yang J, et al. Loss of pleckstrin-2 reverts lethality and vascular occlusions in JAK2V617F-positive myeloproliferative neoplasms. J Clin Investig. 2017;128:125–40.
Wautier M-P, El Nemer W, Gane P, Rain J-D, Cartron J-P, Colin Y, et al. Increased adhesion to endothelial cells of erythrocytes from patients with polycythemia vera is mediated by laminin 5 chain and Lu/BCAM. Blood. 2007;110:894–901.
De Grandis M, Cambot M, Wautier M-P, Cassinat B, Chomienne C, Colin Y, et al. JAK2V617F activates Lu/BCAM-mediated red cell adhesion in polycythemia vera through an EpoR-independent Rap1/Akt pathway. Blood. 2013;121:658–65.
Poisson J, Tanguy M, Davy H, Camara F, Mdawar M-BE, Kheloufi M, et al. Erythrocyte-derived microvesicles induce arterial spasms in JAK2V617F myeloproliferative neoplasm. J Clin Invest. 2020;130:2630–43. https://www.jci.org/articles/view/124566/pdf
Passamonti F, Rumi E, Pietra D, Elena C, Boveri E, Arcaini L, et al. A prospective study of 338 patients with polycythemia vera: the impact of JAK2 (V617F) allele burden and leukocytosis on fibrotic or leukemic disease transformation and vascular complications. Leukemia. 2010;24:1574–9.
Carobbio A, Ferrari A, Masciulli A, Ghirardi A, Barosi G, Barbui T. Leukocytosis and thrombosis in essential thrombocythemia and polycythemia vera: a systematic review and meta-analysis. Blood Adv. 2019;3:1729–37.
Ronner L, Podoltsev N, Gotlib J, Heaney ML, Kuykendall AT, O’Connell C, et al. Persistent leukocytosis in polycythemia vera is associated with disease evolution but not thrombosis. Blood. 2020;135:1696–703.
Alvarez-Larrán A, Arellano-Rodrigo E, Reverter JC, Domingo A, Villamor N, Colomer D, et al. Increased platelet, leukocyte, and coagulation activation in primary myelofibrosis. Ann Hematol. 2008;87:269–76.
Wang W, Liu W, Fidler T, Wang Y, Tang Y, Woods B, et al. Macrophage inflammation, erythrophagocytosis, and accelerated atherosclerosis in Jak2 V617F mice. Circ Res. 2018;123:e35–47. https://www.ahajournals.org/doi/10.1161/CIRCRESAHA.118.313283
Falanga A, Marchetti M, Evangelista V, Vignoli A, Licini M, Balicco M, et al. Polymorphonuclear leukocyte activation and hemostasis in patients with essential thrombocythemia and polycythemia vera. Blood. 2000;96:7.
Marchetti M, Castoldi E, Spronk HMH, van Oerle R, Balducci D, Barbui T, et al. Thrombin generation and activated protein C resistance in patients with essential thrombocythemia and polycythemia vera. Blood. 2008;112:4061–8.
Guy A, Favre S, Labrouche-Colomer S, Deloison L, Gourdou-Latyszenok V, Renault M-A, et al. High circulating levels of MPO-DNA are associated with thrombosis in patients with MPN. Leukemia. 2019;33:2544–8.
Gupta N, Edelmann B, Schnoeder TM, Saalfeld FC, Wolleschak D, Kliche S, et al. JAK2-V617F activates β1-integrin-mediated adhesion of granulocytes to vascular cell adhesion molecule 1. Leukemia. 2017;31:1223–6.
Edelmann B, Gupta N, Schnöder TM, Oelschlegel AM, Shahzad K, Goldschmidt J, et al. JAK2-V617F promotes venous thrombosis through β1/β2 integrin activation. J Clin Invest. 2018;128:4359–71. http://www.jci.org/articles/view/90312
Marin Oyarzún CP, Carestia A, Lev PR, Glembotsky AC, Castro Ríos MA, Moiraghi B, et al. Neutrophil extracellular trap formation and circulating nucleosomes in patients with chronic myeloproliferative neoplasms. Sci Rep. 2016;6:38738.
Wolach O, Sellar RS, Martinod K, Cherpokova D, McConkey M, Chappell RJ, et al. Increased neutrophil extracellular trap formation promotes thrombosis in myeloproliferative neoplasms. Sci Transl Med. 2018;10:eaan8292. https://stm.sciencemag.org/content/10/436/eaan8292
Craver BM, Ramanathan G, Hoang S, Chang X, Mendez Luque LF, Brooks S, et al. N-acetylcysteine inhibits thrombosis in a murine model of myeloproliferative neoplasm. Blood Adv. 2020;4:312–21.
Boulanger CM, Loyer X, Rautou P-E, Amabile N. Extracellular vesicles in coronary artery disease. Nat Rev Cardiol. 2017;14:259–72.
Charpentier A, Lebreton A, Rauch A, Bauters A, Trillot N, Nibourel O, et al. Microparticle phenotypes are associated with driver mutations and distinct thrombotic risks in essential thrombocythemia. Haematologica. 2016;101:e365–8.
Duchemin J, Ugo V, Ianotto J-C, Lecucq L, Mercier B, Abgrall J-F. Increased circulating procoagulant activity and thrombin generation in patients with myeloproliferative neoplasms. Thromb Res. 2010;126:238–42.
Marchetti M, Tartari CJ, Russo L, Panova-Noeva M, Leuzzi A, Rambaldi A, et al. Phospholipid-dependent procoagulant activity is highly expressed by circulating microparticles in patients with essential thrombocythemia. Am J Hematol. 2014;89:68–73.
Trappenburg MC, van Schilfgaarde M, Marchetti M, Spronk HM, Cate HT, Leyte A, et al. Elevated procoagulant microparticles expressing endothelial and platelet markers in essential thrombocythemia. Haematologica. 2009;94:911–8.
Moles-Moreau M-P, Ternisien C, Tanguy-Schmidt A, Boyer F, Gardembas M, Dib M, et al. Flow cytometry-evaluated platelet CD36 expression, reticulated platelets and platelet microparticles in essential thrombocythaemia and secondary thrombocytosis. Thromb Res. 2010;126:e394–6.
Kissova J, Ovesna P, Bulikova A, Zavřelova J, Penka M. Increasing procoagulant activity of circulating microparticles in patients with Philadelphia-negative myeloproliferative neoplasms: a single-centre experience. Blood Coagul Fibrinolysis. 2015;26:448–53.
Zhang W, Qi J, Zhao S, Shen W, Dai L, Han W, et al. Clinical significance of circulating microparticles in Ph- myeloproliferative neoplasms. Oncol Lett. 2017;14:2531–6.
Baccouche H, Jemaa MB, Chakroun A, Chadi S, Mahjoub S, Sfar I, et al. The evaluation of the relevance of thrombin generation and procoagulant activity in thrombotic risk assessment in BCR-ABL-negative myeloproliferative neoplasm patients. Int J Lab Hematol. 2017;39:502–7.
Tong D, Yu M, Guo L, Li T, Li J, Novakovic VA, et al. Phosphatidylserine-exposing blood and endothelial cells contribute to the hypercoagulable state in essential thrombocythemia patients. Ann Hematol. 2018;97:605–16.
Wieczorek I, MacGregor IR, Prescott RJ, Ludlam CA. The fibrinolytic system and proteins C and S in treated polycythaemia rubra vera. Blood Coagul Fibrinolysis. 1992;3:823–6.
Bucalossi A, Marotta G, Bigazzi C, Galieni P, Dispensa E. Reduction of antithrombin III, protein C, and protein S levels and activated protein C resistance in polycythemia vera and essential thrombocythemia patients with thrombosis. Am J Hematol. 1996;52:14–20.
Cella G, Marchetti M, Vianello F, Panova-Noeva M, Vignoli A, Russo L, et al. Nitric oxide derivatives and soluble plasma selectins in patients with myeloproliferative neoplasms. Thromb Haemost. 2010;104:151–6.
Belotti A, Elli E, Speranza T, Lanzi E, Pioltelli P, Pogliani E. Circulating endothelial cells and endothelial activation in essential thrombocythemia: results from CD146+ immunomagnetic enrichment—flow cytometry and soluble E-selectin detection. Am J Hematol. 2011;87:319–20.
Torres C, Fonseca AM, Leander M, Matos R, Morais S, Campos M, et al. Circulating endothelial cells in patients with venous thromboembolism and myeloproliferative neoplasms. PLoS One. 2013;8:e81574.
Sozer S, Fiel MI, Schiano T, Xu M, Mascarenhas J, Hoffman R. The presence of JAK2V617F mutation in the liver endothelial cells of patients with Budd-Chiari syndrome. Blood. 2009;113:5246–9.
Rosti V, Villani L, Riboni R, Poletto V, Bonetti E, Tozzi L, et al. Spleen endothelial cells from patients with myelofibrosis harbor the JAK2V617F mutation. Blood. 2013;121:360–8.
Guy A, Gourdou-Latyszenok V, Lay NL, Peghaire C, Kilani B, Dias JV, et al. Vascular endothelial cell expression of JAK2V617F is sufficient to promote a pro-thrombotic state due to increased P-selectin expression. Haematologica. 2019;104:70–81.
Guadall A, Lesteven E, Letort G, Awan Toor S, Delord M, Pognant D, et al. Endothelial cells harbouring the JAK2V617F mutation display pro-adherent and pro-thrombotic features. Thromb Haemost. 2018;118:1586–99.
Pósfai É, Marton I, Borbényi Z, Nemes A. Myocardial infarction as a thrombotic complication of essential thrombocythemia and polycythemia vera. Anatol J Cardiol. 2016;16:397–402.
Larsen AI, Galbraith PD, Ghali WA, Norris CM, Graham MM, Knudtson ML. Characteristics and outcomes of patients with acute myocardial infarction and angiographically normal coronary arteries. Am J Cardiol. 2005;95:261–3.
Agewall S, Beltrame JF, Reynolds HR, Niessner A, Rosano G, Caforio ALP, et al. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur Heart J. 2017;38:143–53.
Neunteufl T, Heher S, Stefenelli T, Pabinger I, Gisslinger H. Endothelial dysfunction in patients with polycythaemia vera. Br J Haematol. 2001;115:354–9.
Hasselbalch HC. Perspectives on the impact of JAK-inhibitor therapy upon inflammation-mediated comorbidities in myelofibrosis and related neoplasms. Expert Rev Hematol. 2014;7:203–16.
Vrtovec M, Anzic A, Zupan IP, Zaletel K, Blinc A. Carotid artery stiffness, digital endothelial function, and coronary calcium in patients with essential thrombocytosis, free of overt atherosclerotic disease. Radio Oncol. 2017;51:203–10.
Akpan IJ, Stein BL. Splanchnic vein thrombosis in the myeloproliferative neoplasms. Curr Hematol Malig Rep. 2018;13:183–90.
How J, Trinkaus KM, Oh ST. Distinct clinical, laboratory and molecular features of myeloproliferative neoplasm patients with splanchnic vein thrombosis. Br J Haematol. 2018;183:310–3.
Smalberg JH, Arends LR, Valla DC, Kiladjian J-J, Janssen HLA, Leebeek FWG. Myeloproliferative neoplasms in Budd-Chiari syndrome and portal vein thrombosis: a meta-analysis. Blood. 2012;120:4921–8.
Kiladjian J-J, Cervantes F, Leebeek FWG, Marzac C, Cassinat B, Chevret S, et al. The impact of JAK2 and MPL mutations on diagnosis and prognosis of splanchnic vein thrombosis: a report on 241 cases. Blood. 2008;111:4922–9.
Rosenberg RD, Aird WC. Vascular-bed–specific hemostasis and hypercoagulable states. N Engl J Med. 1999;340:1555–64.
Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res. 2007;100:174–90.
Poisson J, Hilscher MB, Tanguy M, Hammoutene A, Boulanger CM, Villeval J-L, et al. Endothelial JAK2V617F does not enhance liver lesions in mice with Budd-Chiari syndrome. J Hepatol. 2018;68:1086–7.
Piaggio G, Rosti V, Corselli M, Bertolotti F, Bergamaschi G, Pozzi S, et al. Endothelial colony-forming cells from patients with chronic myeloproliferative disorders lack the disease-specific molecular clonality marker. Blood. 2009;114:3127–30.
Teofili L, Martini M, Iachininoto MG, Capodimonti S, Nuzzolo ER, Torti L, et al. Endothelial progenitor cells are clonal and exhibit the JAK2V617F mutation in a subset of thrombotic patients with Ph-negative myeloproliferative neoplasms. Blood. 2011;117:2700–7.
Guy A, Danaee A, Paschalaki K, Boureau L, Rivière E, Etienne G, et al. Absence of JAK2V617F mutated endothelial colony-forming cells in patients with JAK2V617F myeloproliferative neoplasms and splanchnic vein thrombosis. Hemasphere. 2020;4:e364.
Ataga KI, Kutlar A, Kanter J, Liles D, Cancado R, Friedrisch J, et al. Crizanlizumab for the prevention of pain crises in sickle cell disease. N Engl J Med. 2017;376:429–39.
Lapponi MJ, Carestia A, Landoni VI, Rivadeneyra L, Etulain J, Negrotto S, et al. Regulation of neutrophil extracellular trap formation by anti-inflammatory drugs. J Pharmacol Exp Therapeutics. 2013;345:430–7.
Perdomo J, Leung HHL, Ahmadi Z, Yan F, Chong JJH, Passam FH, et al. Neutrophil activation and NETosis are the major drivers of thrombosis in heparin-induced thrombocytopenia. Nat Commun. 2019;10:1322.
De Meyer SF, Suidan GL, Fuchs TA, Monestier M, Wagner DD. Extracellular chromatin is an important mediator of ischemic stroke in mice. Arterioscler Thromb Vasc Biol. 2012;32:1884–91.
Santilli F, Romano M, Recchiuti A, Dragani A, Falco A, Lessiani G, et al. Circulating endothelial progenitor cells and residual in vivo thromboxane biosynthesis in low-dose aspirin-treated polycythemia vera patients. Blood. 2008;112:1085–90.
Tan X, Shi J, Fu Y, Gao C, Yang X, Li J, et al. Role of erythrocytes and platelets in the hypercoagulable status in polycythemia vera through phosphatidylserine exposure and microparticle generation. Thromb Haemost. 2013;109:1025–32.
Dienava-Verdoold I, Marchetti MR, te Boome LCJ, Russo L, Falanga A, Koene HR, et al. Platelet-mediated proteolytic down regulation of the anticoagulant activity of protein S in individuals with haematological malignancies. Thromb Haemost. 2012;107:468–76.
Alonci A, Allegra A, Bellomo G, Penna G, D’Angelo A, Quartarone E, et al. Evaluation of circulating endothelial cells, VEGF and VEGFR2 serum levels in patients with chronic myeloproliferative diseases. Hematol Oncol. 2008;26:235–9.
Treliński J, Wierzbowska A, Krawczyńska A, Sakowicz A, Pietrucha T, Smolewski P, et al. Plasma levels of angiogenic factors and circulating endothelial cells in essential thrombocythemia: correlation with cytoreductive therapy and JAK2–V617F mutational status. Leuk Lymphoma. 2010;51:1–7.
Shi K, Zhao W, Chen Y, Ho W, Yang P, Zhao Z. Cardiac hypertrophy associated with myeloproliferative neoplasms in JAK2V617F transgenic mice. J Hematol Oncol. 2014;7:25.
Acknowledgements
The authors are especially thankful to Pierre Emmanuel Rautou, Martine Jandrot Perrus, Jean Luc Villeval, William Vainchenker, and the French Intergroup Myeloproliferative (FIM) network. The authors received research grants from ANR-DFG JAKPOT (no. ANR-14-CE35-0022-02), INSERM, Force Hemato, The Fondation Bettencourt Schueller, and the Aquitaine Region.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
AG and JP have nothing to disclose. CJ consulted for Novartis and received funding for travel and accommodation expenses from Novartis.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guy, A., Poisson, J. & James, C. Pathogenesis of cardiovascular events in BCR-ABL1-negative myeloproliferative neoplasms. Leukemia 35, 935–955 (2021). https://doi.org/10.1038/s41375-021-01170-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-021-01170-z
This article is cited by
-
Cancer-associated thrombosis in hematologic malignancies
International Journal of Hematology (2024)
-
Contributions of bone marrow monocytes/macrophages in myeloproliferative neoplasms with JAK2V617F mutation
Annals of Hematology (2023)
-
Endothelial dysfunction and thromboembolism in children, adolescents, and young adults with acute lymphoblastic leukemia
Leukemia (2022)
-
JAK2V617F variant allele frequency >50% identifies patients with polycythemia vera at high risk for venous thrombosis
Blood Cancer Journal (2021)