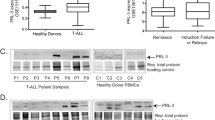
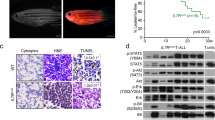
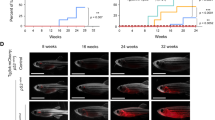
Abstract
T-cell acute lymphoblastic leukemia (T-ALL) is an aggressive malignancy of thymocytes and is largely driven by the NOTCH/MYC pathway. Yet, additional oncogenic drivers are required for transformation. Here, we identify protein tyrosine phosphatase type 4 A3 (PRL3) as a collaborating oncogenic driver in T-ALL. PRL3 is expressed in a large fraction of primary human T-ALLs and is commonly co-amplified with MYC. PRL3 also synergized with MYC to initiate early-onset ALL in transgenic zebrafish and was required for human T-ALL growth and maintenance. Mass-spectrometry phosphoproteomic analysis and mechanistic studies uncovered that PRL3 suppresses downstream T-cell phosphorylation signaling pathways, including those modulated by VAV1, and subsequently suppresses apoptosis in leukemia cells. Taken together, our studies have identified new roles for PRL3 as a collaborating oncogenic driver in human T-ALL and suggest that therapeutic targeting of the PRL3 phosphatase will likely be a useful treatment strategy for T-ALL.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Pui CH, Campana D, Pei D, Bowman WP, Sandlund JT, Kaste SC, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360:2730–41.
Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354:166–78.
Belver L, Ferrando A. The genetics and mechanisms of T cell acute lymphoblastic leukaemia. Nat Rev Cancer. 2016;16:494–507.
Gianni F, Belver L, Ferrando A. The genetics and mechanisms of T-cell acute lymphoblastic leukemia. Cold Spring Harb Perspect Med. 2020;10:a035246.
Weng AP, Millholland JM, Yashiro-Ohtani Y, Arcangeli ML, Lau A, Wai C, et al. c-Myc is an important direct target of Notch1 in T-cell acute lymphoblastic leukemia/lymphoma. Genes Dev. 2006;20:2096–109.
Herranz D, Ambesi-Impiombato A, Palomero T, Schnell SA, Belver L, Wendorff AA, et al. A NOTCH1-driven MYC enhancer promotes T cell development, transformation and acute lymphoblastic leukemia. Nat Med. 2014;20:1130–7.
Ortega M, Bhatnagar H, Lin AP, Wang L, Aster JC, Sill H, et al. A microRNA-mediated regulatory loop modulates NOTCH and MYC oncogenic signals in B- and T-cell malignancies. Leukemia. 2015;29:968–76.
Marks DI, Paietta EM, Moorman AV, Richards SM, Buck G, Dewald G, et al. T-cell acute lymphoblastic leukemia in adults: clinical features, immunophenotype, cytogenetics, and outcome from the large randomized prospective trial (UKALLXII/ECOG 2993). Blood. 2009;114:5136–45.
Petit A, Trinquand A, Chevret S, Ballerini P, Cayuela JM, Grardel N, et al. Oncogenetic mutations combined with MRD improve outcome prediction in pediatric T-cell acute lymphoblastic leukemia. Blood. 2018;131:289–300.
Ko RH, Ji L, Barnette P, Bostrom B, Hutchinson R, Raetz E, et al. Outcome of patients treated for relapsed or refractory acute lymphoblastic leukemia: a therapeutic advances in childhood leukemia consortium study. J Clin Oncol. 2010;28:648–54.
Schrappe M, Hunger SP, Pui CH, Saha V, Gaynon PS, Baruchel A, et al. Outcomes after induction failure in childhood acute lymphoblastic leukemia. N Engl J Med. 2012;366:1371–81.
Trinquand A, Dos Santos NR, Quang CT, Rocchetti F, Zaniboni B, Belhocine M, et al. Triggering the TCR developmental checkpoint activates a therapeutically targetable tumor suppressive pathway in T-cell leukemia. Cancer Discov. 2016;6:973–85.
Bollu LR, Mazumdar A, Savage MI, Brown PH. Molecular pathways: targeting protein tyrosine phosphatases in cancer. Clin Cancer Res. 2017;23:2136–42.
Saha S, Bardelli A, Buckhaults P, Velculescu VE, Rago C, St. Croix B, et al. A phosphatase associated with metastasis of colorectal cancer. Science. 2001;294:1343–6.
Li ZR, Wang Z, Zhu BH, He YL, Peng JS, Cai SR, et al. Association of tyrosine PRL-3 phosphatase protein expression with peritoneal metastasis of gastric carcinoma and prognosis. Surg Today. 2007;37:646–51.
Zhao WB, Li Y, Liu X, Zhang LY, Wang X. Evaluation of PRL-3 expression, and its correlation with angiogenesis and invasion in hepatocellular carcinoma. Int J Mol Med. 2008;22:187–92.
Radke I, Götte M, Kersting C, Mattsson B, Kiesel L, Wülfing P. Expression and prognostic impact of the protein tyrosine phosphatases PRL-1, PRL-2, and PRL-3 in breast cancer. Br J Cancer. 2006;95:347–54.
Fiordalisi JJ, Keller PJ, Cox AD. PRL tyrosine phosphatases regulate Rho family GTPases to promote invasion and motility. Cancer Res. 2006;66:3153–61.
Liang F, Liang J, Wang WQ, Sun JP, Udho E, Zhang ZY. PRL3 promotes cell invasion and proliferation by down-regulation of Csk leading to Src activation. J Biol Chem. 2007;282:5413–9.
Kozlov G, Cheng J, Ziomek E, Banville D, Gehring K, Ekiel I. Structural insights into molecular function of the metastasis-associated phosphatase PRL-3. J Biol Chem. 2004;279:11882–9.
Rios P, Li X, Köhn M. Molecular mechanisms of the PRL phosphatases. FEBS J. 2013;280:505–24.
Ariës IM, Bodaar K, Karim SA, Chonghaile TN, Hinze L, Burns MA, et al. PRC2 loss induces chemoresistance by repressing apoptosis in T cell acute lymphoblastic leukemia. J Exp Med. 2018;215:3094–114.
Burns MA, Liao ZW, Yamagata N, Pouliot GP, Stevenson KE, Neuberg DS, et al. Hedgehog pathway mutations drive oncogenic transformation in high-risk T-cell acute lymphoblastic leukemia. Leukemia. 2018;32:2126–37.
Blackburn JS, Liu S, Raiser DM, Martinez SA, Feng H, Meeker ND, et al. Notch signaling expands a pre-malignant pool of T-cell acute lymphoblastic leukemia clones without affecting leukemia-propagating cell frequency. Leukemia. 2012;26:2069–78.
Garcia EG, Iyer S, Garcia SP, Loontiens S, Sadreyev RI, Speleman F, et al. Cell of origin dictates aggression and stem cell number in acute lymphoblastic leukemia. Leukemia. 2018;32:1860–5.
Wilsbacher JL, Moores SL, Brugge JS. An active form of Vav1 induces migration of mammary epithelial cells by stimulating secretion of an epidermal growth factor receptor ligand. Cell Commun Signal. 2006;4:1–13.
Borga C, Park G, Foster C, Burroughs-Garcia J, Marchesin M, Shah R, et al. Simultaneous B and T cell acute lymphoblastic leukemias in zebrafish driven by transgenic MYC: implications for oncogenesis and lymphopoiesis. Leukemia. 2019;33:333–47.
Borga C, Foster CA, Iyer S, Garcia SP, Langenau DM, Frazer JK. Molecularly distinct models of zebrafish Myc-induced B cell leukemia. Leukemia 2018;33:559–62.
Hara J, Benedict SH, Champagne E, Mak TW, Minden M, Gelfand EW. Comparison of T cell receptor α, β, and γ gene rearrangement and expression in T cell acute lymphoblastic leukemia. J Clin Investig. 1988;81:989–96.
Bajnok A, Ivanova M, Rigó J, Toldi G. The distribution of activation markers and selectins on peripheral T lymphocytes in preeclampsia. Mediators Inflamm. 2017;8045161.
Haubert D, Li J, Saveliev A, Calzascia T, Sutter E, Metzler B, et al. Vav1 GEF activity is required for T cell mediated allograft rejection. Transpl Immunol. 2012;26:212–9.
Wu J, Katzav S, Weiss A. A functional T-cell receptor signaling pathway is required for p95vav activity. Mol Cell Biol. 1995;15:4337–46.
Gulbins E, Coggeshall KM, Baier G. Tyrosine kinase-stimulated guanine nucleotide exchange activity of Vav in T cell activation. Science. 1993;260:822–5.
Lazer G, Pe’er L, Farago M, Machida K, Mayer BJ, Katzav S. Tyrosine residues at the carboxyl terminus of Vav1 play an important role in regulation of its biological activity. J Biol Chem. 2010;285:23075–85.
Wardenburg JB, Fu C, Jackman JK, Flotow H, Wilkinson SE, Williams DH, et al. Phosphorylation of SLP-76 by the ZAP-70 protein-tyrosine kinase is required for T-cell receptor function. J Biol Chem. 1996;271:19641–4.
Bustelo XR. Vav proteins, adaptors and cell signaling. Oncogene. 2001;20:6372–81.
Gulbins E, Coggeshall KM, Baier G, Telford D, Langlet C, Baier-Bitterlich G, et al. Direct stimulation of Vav guanine nucleotide exchange activity for Ras by phorbol esters and diglycerides. Mol Cell Biol. 1994;14:4749–58.
Helou YA, Petrashen AP, Salomon AR. Vav1 regulates T cell activation through a feedback mechanism and crosstalk between the T cell receptor and CD28. J Proteome Res. 2015;176:2963–75.
Aghazadeh B, Lowry WE, Huang XY, Rosen MK. Structural basis for relief of autoinhibition of the Dbl homology domain of proto-oncogene Vav by tyrosine phosphorylation. Cell. 2000;102:625–33.
Kobayashi M, Chen S, Gao R, Bai Y, Zhang ZY, Liu Y. Phosphatase of regenerating liver in hematopoietic stem cells and hematological malignancies. Cell Cycle. 2014;13:2827–35.
Zimmerman MW, Homanics GE, Lazo JS. Targeted Deletion of the metastasis-associated phosphatase Ptp4a3 (PRL-3) suppresses murine colon cancer. PLoS ONE. 2013;8:e58300.
Wei M, Haney MG, Rivas DR, Blackburn JS. Protein tyrosine phosphatase 4A3 (PTP4A3/PRL-3) drives migration and progression of T-cell acute lymphoblastic leukemia in vitro and in vivo. Oncogenesis. 2020;9:6.
Nicholson JM, Cimini D. Cancer karyotypes: survival of the fittest. Front Oncol. 2013;3:148.
Peterson EJ, Maltzman JS, Koretzky GA. T-cell activation and tolerance. In: Robert R. Rich editor. Clinical Immunology: Principles and Practice, 4th ed. USA: Elsevier Inc; 2013;160–71.
Nagasawa K, Howatson A, Mak TW. Induction of human malignant T‐lymphoblastic cell lines MOLT‐3 and jurkat by 12‐O‐tetradecanoylphorbol‐13‐acetate: biochemical, physical, and morphological characterization. J Cell Physiol. 1981;109:181–92.
Wang B, Kishihara K, Zhang D, Sakamoto T, Nomoto K. Transcriptional regulation of a receptor protein tyrosine phosphatase gene hPTP-J by PKC-mediated signaling pathways in Jurkat and Molt-4 T lymphoma cells. Biochim Biophys Acta—Mol Cell Res. 1999;1450:331–40.
Abate F, Da Silva-Almeida AC, Zairis S, Robles-Valero J, Couronne L, Khiabanian H, et al. Activating mutations and translocations in the guanine exchange factor VAV1 in peripheral T-cell lymphomas. Proc Natl Acad Sci USA. 2017;114:764–9.
Robles-Valero J, Lorenzo-Martín LF, Menacho-Márquez M, Fernández-Pisonero I, Abad A, Camós M, et al. A paradoxical tumor-suppressor role for the Rac1 exchange factor Vav1 in T cell acute lymphoblastic leukemia. Cancer Cell. 2017;32:608–23.
Acknowledgements
We thank Christina Luo, Hiranmayi Ravichandran, Rachel Servis, and Ravi Mylvaganam for technical assistance. We thank Drs. Finola Moore and Riadh Lobbardi for helpful discussion and thoughtful review of this manuscript. This work is supported by NIH grant R01CA211734 (DML), R37CA227656 (JSB), CA193651 (AG), the MGH Research Scholar Award (DML), Alex Lemonade Stand Foundation (JSB), the V Foundation for Cancer Research (AG), an Investigatorship from Boston Children’s Hospital (AG), the Research Foundation Flanders (PVV, TT, SL), ‘Kom op tegen Kanker’ (Stand up to Cancer; SL), and the Ghent University Special Research Fund (PVV and TT). Flow cytometry services were supported by MGH Pathology CNY Flow Cytometry Core shared instrumentation grant 1S10RR023440-01A1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Garcia, E.G., Veloso, A., Oliveira, M.L. et al. PRL3 enhances T-cell acute lymphoblastic leukemia growth through suppressing T-cell signaling pathways and apoptosis. Leukemia 35, 679–690 (2021). https://doi.org/10.1038/s41375-020-0937-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-020-0937-3
This article is cited by
-
The FACT-targeted drug CBL0137 enhances the effects of rituximab to inhibit B-cell non-Hodgkin’s lymphoma tumor growth by promoting apoptosis and autophagy
Cell Communication and Signaling (2023)
-
Super-enhancer-driven TOX2 mediates oncogenesis in Natural Killer/T Cell Lymphoma
Molecular Cancer (2023)
-
Proteomic profiling of plasma exosomes from patients with B-cell acute lymphoblastic leukemia
Scientific Reports (2022)