Abstract
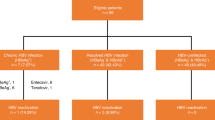
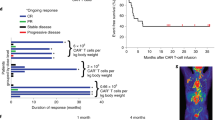
Chimeric antigen receptor (CAR)-T-cell is a safe and effective therapy of B-cell cancers but it is unknown if this is so in persons with prior hepatitis B virus (HBV) infection. We studied 70 subjects with advanced B-cell cancers receiving CAR-T-cell therapy, 12 of whom had chronic HBV-infection (HBsAg positive) and 29 with resolved HBV-infection (HBsAg negative and anti-HBc positive). Safety and efficacy were compared with 29 subjects without HBV-infection. HBV was reactivated in 2 subjects with chronic HBV-infection and 1 with resolved HBV-infection. There was no HBV-related hepatitis flare. Responses to CAR-T-cell therapy in the three cohorts were not significantly different. There was no significant difference in the incidence or severity of cytokine release syndrome (CRS) and neurologic toxicity between the cohorts. Our data suggest that chronic and resolved HBV-infection do not affect the safety and efficacy of CAR-T-cell therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Raje N, Berdeja J, Lin Y, Siegel D, Jagannath S, Madduri D, et al. Anti-BCMA CAR T-Cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med. 2019;380:1726–37.
Tsukune Y, Sasaki M, Odajima T, Sunami K, Takei T, Moriuchi Y. et al.Incidence and risk factors of hepatitis B virus reactivation in patients with multiple myeloma in an era with novel agents: a nationwide retrospective study in Japan. Blood Cancer J. 2017;7:631.
Seto WK, Chan TS, Hwang YY, Wong DK, Fung J, Liu KS, et al. Hepatitis B reactivation in patients with previous hepatitis B virus exposure undergoing rituximab-containing chemotherapy for lymphoma: a prospective study. J Clin Oncol. 2014;32:3736–43.
Cheng CL, Huang SC, Chen JH, Wei CH, Fang WQ, Su TH, et al. Hepatitis B surface antigen positivity is an independent unfavorable prognostic factor in diffuse large B-cell lymphoma in the rituximab era. Oncologist. 2020. [Epub ahead of print].
European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–98.
Shin EC, Sung PS, Park SH. Immune responses and immunopathology in acute and chronic viral hepatitis. Nat Rev Immunol. 2016;16:509–23.
Yan Z, Cao J, Cheng H, Qiao J, Zhang H, Wang Y, et al. A combination of humanised anti-CD19 and anti-BCMA CAR T cells in patients with relapsed or refractory multiple myeloma: a single-arm, phase 2 trial. Lancet Haematol. 2019;6:e521–e9.
Perrillo RP, Martin P, Lok AS. Preventing hepatitis B reactivation due to immunosuppressive drug treatments. JAMA 2015;313:1617–8.
Park JW, Kwak KM, Kim SE, Jang MK, Suk KT, Kim DJ, et al. Comparison of the long-term efficacy between entecavir and tenofovir in treatment- naive chronic hepatitis B patients. BMC Gastroenterol 2017;17:39.
Acknowledgements
Supported in part by grants 81930005, 81970159, 81871263, 81700177, and 81600145 from National Natural Science Foundation of China, grant BK20160232 from Nature Science Foundation of Jiangsu Province, grant 2016M590508 from China Postdoctoral Science Foundation funded project, grant 2015-WSW-058 from Foundation of Jiangsu Province Six Talents Peak, and grant LGY2018084 from Foundation of Jiangsu Province Six-one Project. RPG acknowledges support from the National Institute of Health Research (NIHR) Biomedical Research Centre funding scheme.
Author information
Authors and Affiliations
Contributions
KX, FZ, YW, and YL designed the study. All the authors did acquisition, analysis, or interpretation of data. YW, YL, KQ, JQ, HC, XT, and JG drafted the paper. RPG, KX, FZ, ZY, BP, and JQ performed Critical revision of the paper for important intellectual content. YW, YL, HC, KQ, ZY, and XT did Statistical analysis. MS, GJ, JC, and JZ provided administrative, technical, or material support.
Corresponding authors
Ethics declarations
Conflict of interest
GJ is an employee of iCARTAB Biomedical Co Ltd. The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, Y., Liu, Y., Tan, X. et al. Safety and efficacy of chimeric antigen receptor (CAR)-T-cell therapy in persons with advanced B-cell cancers and hepatitis B virus-infection. Leukemia 34, 2704–2707 (2020). https://doi.org/10.1038/s41375-020-0936-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-020-0936-4
This article is cited by
-
Hepatitis B virus reactivation associated with CAR T-cell therapy
Holistic Integrative Oncology (2024)
-
HBV reactivation in patients with chronic or resolved HBV infection following BCMA-targeted CAR-T cell therapy
Bone Marrow Transplantation (2023)
-
Infectious complications, immune reconstitution, and infection prophylaxis after CD19 chimeric antigen receptor T-cell therapy
Bone Marrow Transplantation (2022)