Abstract
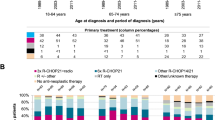
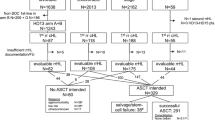
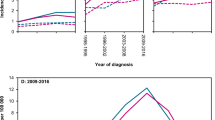
Population-based studies of classical Hodgkin lymphoma (cHL) in contemporary clinical practice are scarce. The aim of this nationwide population-based study is to assess trends in primary therapy and relative survival (RS) during 1989–2017. We included 9,985 patients with cHL. Radiotherapy alone was virtually not applied as from 2000 among patients aged 18–69 years with stage I/II disease, following the broader application of chemotherapy combined with radiotherapy. Chemotherapy only was the preferred treatment for patients with stage III/IV disease. Throughout the entire study period, around 20% of patients aged ≥70 years across all disease stages received no anti-neoplastic therapy. The most considerable improvements in 5-year RS were confined to patients aged 18–59 years. Five-year RS for patients with stage I/II disease diagnosed during 2010–2017 was 99%, 98%, 100%, 93%, 84%, and 61% for patients aged 18–29, 30–39, 40–49, 50–59, 60–69, and ≥70 years, respectively. The corresponding estimates for stage III/IV disease were 96%, 92%, 90%, 80%, 58%, and 46%. Collectively, the improvements in survival likely relate to advances in cHL management. These achievements, however, do not seem to translate into significant benefits for patients ≥60 years. Therefore, novel therapies are urgently needed to reduce excess mortality in elderly cHL patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sant M, Allemani C, Tereanu C, De Angelis R, Capocaccia R, Visser O, et al. Incidence of hematologic malignancies in Europe by morphologic subtype: results of the HAEMACARE project. Blood. 2010;116:3724–34.
Harris N, Jaffe E, Stein H, Banks P, Chan J, Cleary M, et al. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84:1361–92.
Posthuma HLA, Zijlstra JM, Visser O, Lugtenburg PJ, Kersten MJ, Dinmohamed AG. Primary therapy and survival among patients with nodular lymphocyte-predominant Hodgkin lymphoma: a population-based analysis in the Netherlands, 1993–2016. Br J Haematol. 2020;189:117–21.
Bonadonna G, Zucali R, Monfardini S, De Lena M, Uslenghi C. Combination chemotherapy of Hodgkin’s disease with adriamycin, bleomycin, vinblastine, and imidazole carboxamide versus MOPP. Cancer. 1975;36:252–9.
Canellos GP, Anderson JR, Propert KJ, Nissen N, Cooper MR, Henderson ES, et al. Chemotherapy of advanced Hodgkin’s disease with MOPP, ABVD or MOPP alternating with ABVD. N Engl J Med. 1992;327:1478–84.
Diehl V, Franklin J, Pfreundschuh M, Lathan B, Paulus U, Hasenclever D, et al. Standard and increased-dose BEACOPP chemotherapy compared with COPP-ABVD for advanced Hodgkin’s disease. N Engl J Med. 2003;348:2386–95.
Engert A, Schiller P, Josting A, Herrmann R, Koch P, Sieber M, et al. Involved-field radiotherapy is equally effective and less toxic compared with extended-field radiotherapy after four cycles of chemotherapy in patients with early-stage unfavorable Hodgkin’s lymphoma: results of the HD8 trial of the German Hodgkin’s Lymphoma Study Group. J Clin Oncol. 2003;21:3601–8.
Straus DJ, Portlock CS, Qin J, Myers J, Zelenetz AD, Moskowitz CH, et al. Results of a prospective randomized clinical trial of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) followed by radiation therapy (RT) versus ABVD alone for stages I, II, and IIIA nonbulky Hodgkin disease. Blood. 2004;104:3483–9.
Viviani S, Zinzani PL, Rambaldi A, Brusamolino E, Levis A, Bonfante V, et al. ABVD versus BEACOPP for Hodgkin’s lymphoma when high-dose salvage is planned. N Engl J Med. 2011;365:203–12.
Carde P, Karrasch M, Fortpied C, Brice P, Khaled H, Casasnovas O, et al. Eight cycles of ABVD versus four cycles of BEACOPPescalated plus four cycles of BEACOPPbaseline in Stage III to IV, international prognostic score >/= 3, high-risk Hodgkin lymphoma: first results of the phase III EORTC 20012 intergroup trial. J Clin Oncol. 2016;34:2028–36.
Barrington SF, Mikhaeel NG, Kostakoglu L, Meignan M, Hutchings M, Mueller SP, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol. 2014;32:3048–58.
Andre M, Girinsky T, Federico M, Reman O, Fortpied C, Gotti M, et al. Early positron emission tomography response–adapted treatment in stage I and II Hodgkin lymphoma: final results of the randomized EORTC/LYSA/FIL H10 Trial. J Clin Oncol. 2017;35:1786–94.
Radford J, Illidge T, Counsell N, Hancock B, Pettengell R, Johnson P, et al. Results of a trial of PET-directed therapy for early-stage Hodgkin’s lymphoma. N Engl J Med. 2015;372:1598–607.
Fuchs M, Goergen H, Kobe C, Kuhnert G, Lohri A, Greil R, et al. Positron emission tomography–guided treatment in early-stage favorable Hodgkin lymphoma: final results of the international, randomized phase III HD16 trial by the German Hodgkin study group. J Clin Oncol. 2019;37:2835–45.
Borchmann P, Goergen H, Kobe C, Lohri A, Greil R, Eichenauer DA, et al. PET-guided treatment in patients with advanced-stage Hodgkin’s lymphoma (HD18): final results of an open-label, international, randomised phase 3 trial by the German Hodgkin Study Group. Lancet. 2017;390:2790–802.
Younes A, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol. 2012;30:2183–9.
Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372:311–9.
Kersten MJ, Driessen J, Zijlstra JM, Plattel WJ, Morschhauser F, Lugtenburg PJ, et al. Combining brentuximab vedotin with dexamethasone, high-dose cytarabine and cisplatin as salvage treatment in relapsed or refractory Hodgkin lymphoma: the phase II HOVON/LLPC Transplant BRaVE study. Haematologica. 2020; https://doi.org/10.3324/haematol.2019.243238. [Epub ahead of print].
Hagenbeek A, Mooij H, Zijlstra J, Lugtenburg P, Van Imhoff G, Nijland M, et al. Phase 1 dose-escalation study of brentuximab-vedotin combined with dexamethasone, high-dose cytarabine and cisplatin, as salvage treatment in relapsed/refractory classical Hodgkin lymphoma: the Transplant BRaVE study. Haematologica. 2018;104:e151–e3.
Beaver J, Ison G, Pazdur R. Reevaluating eligibility criteria-balancing patient protection and participation in oncology trials. N Engl J Med. 2017;376:1504–5.
Bjorkholm M, Weibull CE, Eloranta S, Smedby KE, Glimelius I, Dickman PW. Greater attention should be paid to developing therapies for elderly patients with Hodgkin lymphoma-A population-based study from Sweden. Eur J Haematol. 2018;101:106–14.
Sant M, Minicozzi P, Mounier M, Anderson LA, Brenner H, Holleczek B, et al. Survival for haematological malignancies in Europe between 1997 and 2008 by region and age: results of EUROCARE-5, a population-based study. Lancet Oncol. 2014;15:931–42.
Sjoberg J, Halthur C, Kristinsson SY, Landgren O, Nygell UA, Dickman PW, et al. Progress in Hodgkin lymphoma: a population-based study on patients diagnosed in Sweden from 1973–2009. Blood. 2012;119:990–6.
Glimelius I, Ekberg S, Jerkeman M, Chang ET, Bjorkholm M, Andersson TM, et al. Long-term survival in young and middle-aged Hodgkin lymphoma patients in Sweden 1992–2009-trends in cure proportions by clinical characteristics. Am J Hematol. 2015;90:1128–34.
Bessell EM, Bouliotis G, Armstrong S, Baddeley J, Haynes AP, O’Connor S, et al. Long-term survival after treatment for Hodgkin’s disease (1973–2002): improved survival with successive 10-year cohorts. Br J Cancer. 2012;107:531–6.
Solans M, Serra L, Renart G, Osca-Gelis G, Comas R, Vilardell L, et al. Incidence and survival of Hodgkin lymphoma patients in Girona (Spain) over three decades: a population-based study. Eur J Cancer Prev. 2017;26:S164–S9.
Lagerlof I, Holte H, Glimelius I, Bjorkholm M, Enblad G, Erlanson M, et al. No excess long-term mortality in stage I-IIA Hodgkin lymphoma patients treated with ABVD and limited field radiotherapy. Br J Haematol. 2020;188:685–91.
Schouden LJ, Höppener P, Van den Brandt PA, Knottnerus JA, Jager JJ. Completeness of cancer registration in Limburg, the Netherlands. Int J Epidemiol. 1993;22:369–76.
Linch DC, Winfield D, Goldstone AH, Moir D, Hancock B, McMillan A, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin’s disease: results of a BNLI randomised trial. Lancet. 1993;341:1051–4.
Gallamini A, Tarella C, Viviani S, Rossi A, Patti C, Mulé A, et al. Early chemotherapy intensification with escalated BEACOPP in patients with advanced-stage Hodgkin lymphoma with a positive interim positron emission tomography/computed tomography scan after two ABVD cycles: long-term results of the GITIL/FIL HD 0607 trial. J Clin Oncol. 2018;36:454–62.
Dickman PW, Adami HO. Interpreting trends in cancer patient survival. J Intern Med. 2006;260:103–17.
Ederer F, Heise H. Instructions to IBM 650 programmers in processing survival computations. Methodological note No.10. End Results Evaluation Section. Bethesda MD: National Cancer Institute. 1959.
Liu L, Giusti F, Schaapveld M, Aleman B, Lugtenburg P, Meijnders P, et al. Survival differences between patients with Hodgkin lymphoma treated inside and outside clinical trials. A study based on the EORTC-Netherlands Cancer Registry linked data with 20 years of follow-up. Br J Haematol. 2017;176:65–75.
Evens AM, Antillon M, Aschebrook-Kilfoy B, Chiu BC. Racial disparities in Hodgkin’s lymphoma: a comprehensive population-based analysis. Ann Oncol. 2012;23:2128–37.
Glaser SL, Clarke CA, Chang ET, Yang J, Gomez SL, Keegan TH. Hodgkin lymphoma incidence in California Hispanics: influence of nativity and tumor Epstein-Barr virus. Cancer Causes Control. 2014;25:709–25.
Eichenauer DA, Aleman BMP, Andre M, Federico M, Hutchings M, Illidge T, et al. Hodgkin lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Supplement_4):iv19–iv29.
Johnson P, Federico M, Kirkwood A, Fossa A, Berkahn L, Carella A, et al. Adapted treatment guided by interim PET-CT scan in advanced Hodgkin’s lymphoma. N Engl J Med. 2016;374:2419–29.
Schmitz N, Pfistner B, Sextro M, Sieber M, Carella AM, Haenel M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: a randomised trial. Lancet. 2002;359:2065–71.
Evens AM, Advani RH, Helenowski IB, Fanale M, Smith SM, Jovanovic BD, et al. Multicenter phase II study of sequential brentuximab vedotin and doxorubicin, vinblastine, and dacarbazine chemotherapy for older patients with untreated classical Hodgkin lymphoma. J Clin Oncol. 2018;36:3015–22.
Acknowledgements
The authors would like to thank the registration clerks of the Netherlands Cancer Registry (NCR) for their dedicated data collection. The nationwide population-based NCR is maintained and hosted by the Netherlands Comprehensive Cancer Organisation (IKNL). This work was supported by research funding from Stichting SHOW (grant to Julia Driessen).
Author information
Authors and Affiliations
Contributions
AGD designed the study; JD analyzed the data; AGD provided statistical support; OV collected the data; JD wrote the manuscript with contributions from all authors, who also interpreted the data, and read, commented on, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There is no financial support for this work that could have influenced the outcomes described in the manuscript. However, particular authors report a potential conflict of interest, which is described below. MJK: Millennium/Takeda: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Roche: Honoraria, Research Funding; Gilead: Honoraria; Kite Pharma: Honoraria; Novartis: Honoraria. PJL: Millennium/Takeda: Consultancy, Research Funding; Servier: Consultancy, Research Funding; Roche: Consultancy; BMS: Consultancy; Sandoz: Consultancy; Genmab: Consultancy. JMZ: Consultant/Advisor: Gilead, Roche, Takeda; Honoraria: Gilead, Roche, Takeda, Janssen. AGD: BMS: Research funding. All remaining authors have declared no competing financial interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Driessen, J., Visser, O., Zijlstra, J.M. et al. Primary therapy and relative survival in classical Hodgkin lymphoma: a nationwide population-based study in the Netherlands, 1989–2017. Leukemia 35, 494–505 (2021). https://doi.org/10.1038/s41375-020-0875-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-020-0875-0
This article is cited by
-
Vincristine-based nanoformulations: a preclinical and clinical studies overview
Drug Delivery and Translational Research (2023)
-
Time trends in primary therapy and relative survival of diffuse large B-cell lymphoma by stage: a nationwide, population-based study in the Netherlands, 1989–2018
Blood Cancer Journal (2022)