Abstract
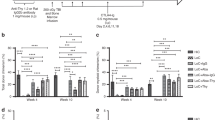
Patients receiving an allogeneic hematopoietic cell transplantation (allo-HCT) after the use of PD-1 inhibitors seem to be at a higher risk of developing acute graft-versus-host disease (aGHVD) through etiopathogenetic mechanisms not fully elucidated. Herein, we investigated the effect of nivolumab administered prior to allo-HCT on the following early T-cell reconstitution and its modulation by the GVHD prophylaxis (tacrolimus/sirolimus vs. posttransplant cyclophosphamide [PTCY]). In all nivolumab-exposed patients we detected circulating nivolumab in plasma for up to 56 days after allo-HCT. This residual nivolumab was able to bind and block PD-1 on T-cells at day 21 after allo-HCT, inducing a T cell activation that was differentially modulated depending on the GVHD prophylactic regimen. Among patients receiving tacrolimus/sirolimus, nivolumab-exposed patients had a higher incidence of severe aGVHD and a more effector T-cell profile compared with anti-PD-1-naïve patients. Conversely, patients receiving PTCY-based prophylaxis showed a similar risk of aGVHD and T-cell profile irrespective of the previous nivolumab exposure. In conclusion, nivolumab persists in plasma after transplantation, binds to allogeneic T cells and generates an increased T-cell activation. This T-cell activation status can be mitigated with the use of PTCY, thus reducing the risk of aGVHD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372:311–9.
Armand P, Engert A, Younes A, Fanale M, Santoro A, Zinzani PL, et al. Nivolumab for relapsed/refractory classic hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: extended follow-up of the multicohort single-arm phase II CheckMate 205 trial. J Clin Oncol. 2018;36:1428–39.
Blazar BR, Carreno BM, Panoskaltsis-Mortari A, Carter L, Iwai Y, Yagita H, et al. Blockade of programmed death-1 engagement accelerates graft-versus-host disease lethality by an IFN-gamma-dependent mechanism. J Immunol. 2003;171:1272–7.
Holderried Taw, Fraccaroli A, Schumacher M, Heine A, Brossart P, Stelljes M, et al. The role of checkpoint blockade after allogeneic stem cell transplantation in diseases other than Hodgkin’s Lymphoma. Bone Marrow Transpl. 2019;54:1662–7.
Merryman RW, Kim HT, Zinzani PL, Carlo-Stella C, Ansell SM, Perales MA, et al. Safety and efficacy of allogeneic hematopoietic stem cell transplant after PD-1 blockade in relapsed/refractory lymphoma. Blood. 2017;129:1380–8.
Schoch LK, Cooke KR, Wagner-Johnston ND, Gojo I, Swinnen LJ, Imus P, et al. Immune checkpoint inhibitors as a bridge to allogeneic transplantation with post-transplant cyclophosphamide. Blood Adv. 2018;2:2226–9.
Kanakry CG, Coffey DG, Towlerton AM, Vulic A, Storer BE, Chou J, et al. Origin and evolution of the T cell repertoire after post-transplantation cyclophosphamide. JCI Insight. 2016;1:e86252.
Wachsmuth LP, Patterson MT, Eckhaus MA, Venzon DJ, Gress RE, Kanakry CG. Post-transplantation cyclophosphamide prevents graft-versus-host disease by inducing alloreactive T cell dysfunction and suppression. J Clin Investig. 2019;129:2357–73.
Kanakry CG, Ganguly S, Zahurak M, Bolanos-Meade J, Thoburn C, Perkins B, et al. Aldehyde dehydrogenase expression drives human regulatory T cell resistance to post-transplantation cyclophosphamide. Sci Transl Med. 2013;5:211ra157.
Ikegawa S, Meguri Y, Kondo T, Sugiura H, Sando Y, Nakamura M, et al. PTCy ameliorates GVHD by restoring regulatory and effector T-cell homeostasis in recipients with PD-1 blockade. Blood Adv. 2019;3:4081–94.
Luznik L, Bolanos-Meade J, Zahurak M, Chen AR, Smith BD, Brodsky R, et al. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood. 2010;115:3224–30.
Parody R, Lopez-Corral L, Lopez-Godino O, Martinez C, Martino R, Solano C, et al. GvHD prophylaxis with tacrolimus plus sirolimus after reduced intensity conditioning allogeneic transplantation: results of a multicenter study. Bone Marrow Transpl. 2016;51:1524–6.
Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–75.
Ni X, Song Q, Cassady K, Deng R, Jin H, Zhang M, et al. PD-L1 interacts with CD80 to regulate graft-versus-leukemia activity of donor CD8+ T cells. J Clin Investig. 2017;127:1960–77.
Kanakry CG, O’Donnell PV, Furlong T, de Lima MJ, Wei W, Medeot M, et al. Multi-institutional study of post-transplantation cyclophosphamide as single-agent graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation using myeloablative busulfan and fludarabine conditioning. J Clin Oncol. 2014;32:3497–505.
Acknowledgements
The authors thank the patients and healthy individuals from the Vall d’Hebron University Hospital that enrolled in the study, as well as the Cellex Foundation for providing research facilities and equipment. This work was supported by the Instituto de Salud Carlos III Fondo de Investigaciones Sanitarias (FIS16/01433, PB; PI17/00950, MC, and PI17/00943, FB) and co-financed by the European Regional Development Fund (ERDF). A PERIS 2018–2020 grant from the Generalitat de Catalunya (BDNS357800, PB), Asociación Española Contra el Cáncer (Ideas Semilla 2019, P.B and LABAE18014CRES, MC) and Gilead Fellowships (GLD16/00144, GLD18/00047, FB). MC holds a contract from Ministerio de Ciencia, Innovación y Universidades (RYC-2012–12018).
Author information
Authors and Affiliations
Contributions
Conception and design: JCN, MC, and PB Technical procedures: JCN Sample processing and data collection: ER, IJ, LF, JC, GO, LLP, LG, GI, PR, AP, OS, and CP. Data analysis and interpretation: JCN, SB, DV, FB, MC, and PB. Manuscript writing: All authors. Final approval of manuscript: All authors.
Corresponding author
Ethics declarations
Conflict of interest
PB declares having received honoraria from Amgen, Celgene, Gilead, Incyte, Jazz Pharmaceuticals, MSD, Novartis, Pfizer and Roche, not related with the present article. MC has received research funding from Karyopharm, Pharmacyclics, Roche, Arqule and AstraZeneca, not related with the present article. FB has received research funding and honoraria from Roche, Celgene, Takeda, AstraZeneca, Novartis, Abbie and Janssen, not related with the present article.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Nieto, J.C., Roldán, E., Jiménez, I. et al. Posttransplant cyclophosphamide after allogeneic hematopoietic cell transplantation mitigates the immune activation induced by previous nivolumab therapy. Leukemia 34, 3420–3425 (2020). https://doi.org/10.1038/s41375-020-0851-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-020-0851-8
This article is cited by
-
Safety and efficacy of immune checkpoint inhibitors after allogeneic hematopoietic cell transplantation
Bone Marrow Transplantation (2023)
-
Anti-programmed cell death-1 monoclonal antibody therapy before or after allogeneic hematopoietic cell transplantation for classic Hodgkin lymphoma: a literature review
International Journal of Hematology (2022)
-
Checkpoint Inhibitors and the Changing Face of the Relapsed/Refractory Classical Hodgkin Lymphoma Pathway
Current Oncology Reports (2022)
-
Pretransplant nivolumab further enhanced Treg expansion after posttransplant cyclophosphamide; another aspect for immune tolerance by PTCy after nivolumab
Leukemia (2021)
-
Use of checkpoint inhibitors in patients with lymphoid malignancies receiving allogeneic cell transplantation: a review
Bone Marrow Transplantation (2021)