Abstract
Blast crisis is one of the remaining challenges in chronic myeloid leukemia (CML). Whether additional chromosomal abnormalities (ACAs) enable an earlier recognition of imminent blastic proliferation and a timelier change of treatment is unknown. One thousand five hundred and ten imatinib-treated patients with Philadelphia-chromosome-positive (Ph+) CML randomized in CML-study IV were analyzed for ACA/Ph+ and blast increase. By impact on survival, ACAs were grouped into high risk (+8, +Ph, i(17q), +17, +19, +21, 3q26.2, 11q23, −7/7q abnormalities; complex) and low risk (all other). The presence of high- and low-risk ACAs was linked to six cohorts with different blast levels (1%, 5%, 10%, 15%, 20%, and 30%) in a Cox model. One hundred and twenty-three patients displayed ACA/Ph+ (8.1%), 91 were high risk. At low blast levels (1–15%), high-risk ACA showed an increased hazard to die compared to no ACA (ratios: 3.65 in blood; 6.12 in marrow) in contrast to low-risk ACA. No effect was observed at blast levels of 20–30%. Sixty-three patients with high-risk ACA (69%) died (n = 37) or were alive after progression or progression-related transplantation (n = 26). High-risk ACA at low blast counts identify end-phase CML earlier than current diagnostic systems. Mortality was lower with earlier treatment. Cytogenetic monitoring is indicated when signs of progression surface or response to therapy is unsatisfactory.
Similar content being viewed by others
Introduction
Blast crisis (BC) of Philadelphia-chromosome-positive (Ph+) and/or BCR-ABL1-positive chronic myeloid leukemia (CML) is one of the remaining challenges in the management of the disease. Cytogenetic abnormalities and blast increase represent the most consistent indicators of progression to end-phase CML [1,2,3,4,5,6,7]. End-phase CML comprises early progression with emerging high-risk ACA and late progression with failing hematopoiesis and blast cell proliferation. BC represents the end stage of this evolution. Not all patients dying of CML reach the blast levels defining BC (20% or 30% blasts in blood or marrow) [8,9,10,11]. Once BC has occurred, treatment results are poor. Early allogeneic stem cell transplantation (SCT) might improve prognosis [12, 13]. Whether cytogenetic aberrations allow a timelier change of treatment with better outcome is unknown.
Additional chromosomal abnormalities (ACAs) are thought to result from BCR-ABL1-induced genetic instability and may be causative factors of disease progression [14, 15]. The most frequent ACAs found in BC (+8, a second Ph-chromosome (+Ph), an isochromosome of the long arm of chromosome 17, i.e., i[17q], and +19) were termed major route by Mitelman et al. [1–2]. Major-route ACAs have been associated with shorter survival, if they were detected at diagnosis [16] or if they emerged in the course of disease [17]. A poor prognosis was also observed with 3q26.2 and 11q23 rearrangements and with −7/7q− [18, 19], whereas +8 and +Ph as single aberrations, but not in combination, were not equally associated with poor prognosis [20]. Wang et al. [17] proposed a risk stratification of the six most frequent ACAs into two groups with distinct prognoses (+8, +Ph, −Y with good prognoses and i[17q], −7/7q−, 3q26.2 rearrangements with poor prognoses). Using the same cohort of patients, a risk stratification into three groups was proposed [21] based on BC risk associated with each ACA (high risk: 3q26.2; −7/7q−; i[17q]; complex karyotypes with high-risk ACA. Intermediate 1: +8; +Ph; other single ACA. Intermediate 2: other complex ACA).
Since the prognosis with single changes (+8, +Ph) is controversial, we decided to include these in our evaluation, as well as +21 and +17, which were designated as major route later on [1]. Other ACAs were not so clearly associated with shorter survival. This led to their tentative designation as low-risk ACA.
More recently, clonal chromosomal aberrations (CCAs) found in Ph-negative cells (CCA/Ph−) were reported to also have a negative impact on survival [22, 23].
We here made use of the data of 1551 imatinib-treated chronic phase (CP) patients recruited to CML-study IV, a randomized study comparing imatinib 400 mg with imatinib 800 mg and combinations of imatinib with interferon, simultaneously or sequentially, or low-dose cytarabine [24]. Our aim was to analyze if defined ACA at low blast levels allow an earlier diagnosis of end-phase CML and a timelier change of treatment than current blast thresholds.
Patients and methods
Patients
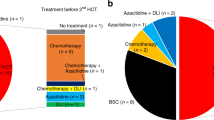
Patient data were derived from the randomized CML-study IV (recruitment 2002–2012) with initial or predominant imatinib treatment [24]. Documentation was done at 3–6-month intervals as previously reported [24]. Risk assignment followed the ELTS (EUTOS Long-Term Survival) score [25]. BC was defined by 30% blasts in blood or marrow. Patient numbers and flow are depicted in the flow chart (Fig. 1a–c).
Cytogenetics
By protocol, cytogenetic analysis was requested every 3–6 months during the early disease phases and every 12 months thereafter, if stable molecular remission (major molecular remission or better, BCR-ABL1 <0.1% on the International Scale, IS) was achieved. In stable molecular remission, intervals between cytogenetic analyses were frequently longer due to patients’ and/or doctors’ requests. A median of four analyses per patient were documented. Cytogenetic analyses were done as described and results were reported according to the international nomenclature (ISCN 2016) [16]. ACAs were evaluated if they were clonal according to the ISCN. Complex karyotypes were defined as three or more concurrent aberrations. High-risk ACAs were defined as the major route ACA +8, +Ph, i[17q], +19, +21, +17 (the ACA most frequently observed in BC) [1], the minor route ACA 3q26.2, 11q23, −7/7q− (less frequently observed, but negative impact on prognosis) [17, 19, 18], and complex karyotypes. Variant translocations and −Y were not considered, as they had no impact on prognosis in our and other studies [16, 26].
CAA/Ph− have not been an objective of this study.
Molecular genetics
Molecular analyses followed the IS methodology and nomenclature [27,28,29,30].
Statistics
For survival analyses, patients were followed up at the start of the diagnosis, at the time of the occurrence of an ACA, or at the time of a blast increase. Patients were censored at the date of last follow-up. Mortality after the advent of blast increases was assessed by Cox models starting at the time of a blast increase. Here the presence of ACA was considered as a time-dependent covariate. P values <5% were considered significant. Due to the explorative character of this work, no adjustment of p values was done and all p values have to be interpreted descriptively. All analyses were performed with SAS 9.3 or R 3.5.1.
Results
Identification of ACA
One thousand five hundred and thirty-six patients with Ph+ CP-CML were analyzed for blast increase and ACA, 1510 patients were cytogenetically evaluable. Patients’ characteristics are shown in Table 1. Median observation time was 8.6 years.
One hundred and twenty-three patients (8.1%) displayed ACA in Ph+ metaphases (Appendix A1). Ninety one (6%) were high-risk ACAs (+8, +Ph, i[17q], +17, +19, +21, 11q23 and 3q26.2 rearrangements, −7/7q abnormalities, complex karyotypes) and 32 (2.1%) were low-risk ACAs (all other). Of the 91 high-risk ACAs, 25 (1.7%) were detected at baseline and 66 (4.4%) emerged in the course of disease 0.5–133 months after diagnosis. The median time to detection of high-risk ACAs was 17 months. Of the 32 low-risk ACAs, 19 (1.3%) were detected at diagnosis and 13 (0.9%) emerged in the course of disease.
Frequencies of ACA are shown in Table 2 grouped according to type (risk level, single, or in combination) and time of emergence (at diagnosis or in the course of disease).
Impact of ACA on survival
Figure 2a–d shows the impact of high- and low-risk ACAs on survival from occurrence of ACA at diagnosis or in the course of disease. Observations were synchronized for the time of emergence of ACA, and ACA detected at diagnosis and emerging in the course of disease were analyzed together. All high-risk ACAs show a negative impact on survival compared to low-risk ACAs, which serve as control. An exception is +8 as a single aberration, which carries a prognosis in between high- and low-risk ACAs (Fig. 2a). Impact of +Ph on survival was equally poor whether it occurred alone or in combination with other abnormalities (Fig. 2b). Chromosome 3, 7, 17, 19, and 21 aberrations were grouped together, as they were rare (Fig. 2d). Individual analyses of these aberrations are shown in Fig. 2e–i. Four-year survival probability after occurrence of high-risk ACA, except +8 alone, was 52.2% (95% confidence interval (CI): 41–66), after occurrence of +8 alone 77% (95% CI: 60–100), and after occurrence of low-risk ACA 87% (95% CI: 75–100).
The left-side panel shows the impact of +8 (a), +Ph (b), complex ACA (c), and of chromosome 3, 7, 17, 19, and 21 aberrations combined (d) on survival in patients with primary imatinib treatment after the emergence of ACA. Suvival after emergence of low-risk ACA in imatinib-treated patients serves as control. The right-side panel shows the impact of rare high-risk ACA of chromosomes 3, 7, 17, 19, and 21 on survival (e–i).
The treatment strategy after emergence of ACA including allogeneic SCT did not differ according to the type of ACA, but patients with high-risk ACA were transplanted more frequently. 42 of the 138 transplantations in CML-study IV (30%) were performed in the 6% of patients with high-risk ACA (Flow chart, Fig. 1c). Two-year survival of 26 patients transplanted in BC or accelerated phase (AP) was 46% (95% CI: 26–63%) and of 13 patients transplanted in CP 77% (95% CI: 44–92%; log-rank test: p = 0.09; phase unknown for 3 of 42 patients).
Correlation of ACA with BC
79 patients developed BC during the observation time. Of the 79 BC patients, 71 were cytogenetically evaluable. 44 BC patients had ACA (61%), in 27 patients no ACAs were reported. Of the 44 BC patients with ACA, 41 (93%) had high-risk and 3 low-risk ACA (Flow chart, Fig. 1b).
Correlation of ACA with blast increase
The close correlation of high-risk ACA with BC and the unfavorable prognosis of patients with high-risk ACA led us to ask if high-risk ACA can anticipate the diagnosis of end-phase CML. We therefore assigned patients, in whom a blast increase was observed in peripheral blood or marrow (at any time), to 6 different blast thresholds (1%, 5%, 10%, 15%, 20%, and 30%). In each of these cohorts high- and low-risk ACAs were considered as time-dependent variables. The number of patients ranged from 224 to 78 in the six cohorts with blast increases of 1% to >30% in the peripheral blood, and from 1033 to 79 in the six cohorts with blast levels of 1% to >30% in the marrow (Table 3). Naturally, the sets of patients who developed higher blast increases later on were subsets of the sets with lower blast increases.
In the corresponding Cox proportional hazards models (Table 3, Fig. 3), we found an increased hazard to die in the presence of high-risk ACA compared to no ACA with hazard ratios of up to 3.65 (95% CI: 2.32–5.75) in the blood (Fig. 3a) and 6.12 (95% CI: 4.1–9.2) in the marrow (Fig. 3b) when only patients with low blast levels of 1–5% were considered. When restricting the cohorts to larger blast increases, the effect of high-risk ACA on the hazard to die decreased. In the last cohorts of patients with blast increases to at least 20% or 30%, no difference between patients with and without high-risk ACA was found (hazard ratio: 0.83, 95% CI: 0.50–2.89). The hazard ratios for low-risk ACA compared to no ACA were increased to much lesser extents than for high-risk ACA.
Hazard ratios for mortality in imatinib-treated patients with high-risk and low-risk ACA were determined in six different (but overlapping) patient groups (blast increase to 1–30%) together with 95% confidence intervals a in peripheral blood and b in bone marrow. The size of the circle correlates with the sample size. Thirty-seven patients with high-risk ACA and four patients with low-risk ACA died. In 34 patients with high-risk ACA, causes of death were known. Thirty-two of these (94%) died of progression, including progression-related transplantation in 21 patients. Two patients died of CML-unrelated causes. Causes of death were unknown in three patients. With low-risk ACA, causes of death were CML related in three patients and unknown in one patient.
Course of disease and causes of death
Thirty-seven patients with high-risk ACA (41%) died (Flow chart, Fig. 1a, c). The causes of death were known for 34 patients and almost exclusively CML related. Thirty-two patients (94%) died of progression, mostly in BC, including 21 after progression-related transplantation (15 in BC, 1 in AP, 2 after loss of cytogenetic remission, 2 because of no molecular response, and 1 with unknown indication). Two patients died of CML-unrelated causes.
Of 54 (59%) living patients with high-risk ACAs, 21 (23% of total) were transplanted, 8 of these in BC, 2 in AP, 9 because of no molecular response, and 2 with unknown indication. Five (5%) non-transplanted patients are alive after progression (Flow chart, Fig. 1a, c). Twenty-eight patients (31%) are alive without documented progression 0–11 years after emergence of high-risk ACA.
Of the four patients with low-risk ACA who died, three died of CML-related (one after SCT) and one of CML-unrelated causes
Discussion
We here report that the combination of high-risk ACA and low-level blasts heralds progression and death by CML. This finding has implications for diagnosis and management of patients in end-phase CML. High-risk ACAs allow to identify CML end-phase at an earlier time than is possible with the current blast thresholds [9, 10]. These patients require a change of therapy and/or more intensive treatments, for example, with ponatinib or allogeneic SCT [9]. A clinically relevant, but in 39 patients not statistically significant difference of 30% 2-year survival suggests that outcome of transplanted patients with high-risk ACA depends on disease stage similar to patients without ACA [12]. Successful treatment may explain, at least in part, the plateau phase of survival curves after 4 years.
High-risk ACA at low blast counts are not an indicator of AP as previously reviewed [31, 32], but a marker of progression and death by CML regardless whether they are present at diagnosis or emerge in the course of disease. Sixty-nine percent of patients with high-risk ACA and low blast levels progressed or died of CML during the observation period. This includes transplanted patients who as a rule were transplanted because of progression. Our findings agree with an earlier observation that patients in AP with ACA and blast increase have a worse outcome [33].
Our data suggest that the appropriate time for a change of treatment is emergence of high-risk ACA rather than waiting for an increase of blasts.
High-risk ACA include, in addition to major route ACA (which were defined solely on the basis of their frequency in BC) [1–2], most notably −7/7q−, 3q26.2, and 11q23 rearrangements and complex karyotypes [16,17,18,19,20,21, 26]. Additional high-risk ACA may surface on continued clinical and cytogenetic scrutiny.
Our definition of high-risk ACA largely agrees with that proposed by others [17, 21], but there are differences. The prognosis with +8 alone, although clearly better than with +8 in combination, is still worse than with low-risk ACA (Fig. 2a). We thus have included +8 alone in the high-risk ACA group. +Ph has an equally unfavorable impact on survival, regardless whether it occurs as a single aberration or in combination (Fig. 2b). In agreement with earlier reports [17, 18], chromosome 19 aberrations exclusively occurred in combination, whereas 3q26.2 was mostly found as a single aberration and rarely at diagnosis [16]. −Y in our analyses had a prognosis not different from no ACA.
Looking at our data in context of the literature [17, 21], a risk stratification of ACA in two risk-groups (high risk with impact on survival and low risk with little or no impact) seems useful for clinical purposes. Due to the limited sample size and the exploratory nature of this work, we would welcome verification of these results by others.
High-risk ACAs were not observed in all BC patients. We cannot rule out that other events such as genetic alterations not detectable by cytogenetics predispose to a blast increase. Gene sequencing detects such alterations [34]. Telomere shortening [35] or increased separase activity [36] may contribute to progression. Likewise, low-risk ACA might include some hitherto unrecognized high-risk ACA as suggested by the three patients with BC and low-risk ACA.
High-risk ACAs were observed in 6% of CP patients, but in 61% of patients who had progressed to BC, whereas low-risk ACAs were observed in 2.1% of CP patients and in 2.8% of patients with BC. This is strong support for a role of high-risk ACA in the evolution of CML and is in line with the hypothesis that BCR-ABL1 predisposes to ACA, which then promote progression. The emergence of high-risk ACA might anticipate and define the point of no return in the evolution of CML indicating non-reversibility by tyrosine kinase inhibitor (TKI). High-risk ACA could emerge as testable cause of non-mutation-related TKI resistance.
A limitation of this study is the follow-up cytogenetics, which have been replaced in many instances by molecular testing increasingly done in the course of the study. Furthermore, most cytogenetic analyses were performed in the first months after diagnosis or when the patients’ conditions were worsening. This might introduce a bias, as patients doing well were usually not analyzed. Also, it is possible that analyses with low numbers of mitoses miss ACA and that the true percentage of patients with ACA is underestimated.
Although the low number of follow-up cytogenetics has limited the direct correlation of ACA with blast increase, it points to the strength of the evidence for the association of high-risk ACA with end-phase CML and survival in the Cox model in spite of missing values.
A correlation with BCR-ABL1 transcript numbers was not conclusive because of too few molecular measurements at early blast increase.
A strength of the study is the size of the cohort as one of the largest of TKI-treated CP-CML patients on whom emergence of ACA and progression to BC were prospectively recorded in parallel over prolonged periods of time. The association of high-risk ACA with progression would not be so obvious in smaller cohorts with shorter observation.
In conclusion, high-risk ACAs are an early marker of CML progression. In the presence of low blast levels, high-risk ACAs indicate death by CML earlier than is possible with standard blast thresholds. An appropriate time for a change of therapy may be emergence of high-risk ACA rather than waiting for an increase of blasts. Cytogenetic monitoring is indicated when signs of progression surface and response to therapy is unsatisfactory.
References
Johansson B, Fioretos T, Mitelman F. Cytogenetic and molecular genetic evolution of chronic myeloid leukemia. Acta Haematol. 2002;107:76–94.
Mitelman F, Levan G, Nilsson PG, Brandt L. Non-random karyotypic evolution in chronic myeloid leukemia. Int J Cancer. 1976;18:24–30.
Sadamori N, Matsunaga M, Yao E, Ichimaru M, Sandberg AA. Chromosomal characteristics of chronic and blastic phases of Ph-positive chronic myeloid leukemia. Cancer Genet Cytogenet. 1985;15:17–24.
Anastasi J, Feng J, Le Beau MM, Larson RA, Rowley JD, Vardiman JW. The relationship between secondary chromosomal abnormalities and blast transformation in chronic myelogenous leukemia. Leukemia. 1995;9:628–33.
Majlis A, Smith TL, Talpaz M, O'Brien S, Rios MB, Kantarjian HM. Significance of cytogenetic clonal evolution in chronic myelogenous leukemia. J Clin Oncol. 1996;14:196–203.
Marktel S, Marin D, Foot N, Szydlo R, Bua M, Karadimitris A, et al. Chronic myeloid leukemia in chronic phase responding to imatinib: the occurrence of additional cytogenetic abnormalities predicts disease progression. Haematologica. 2003;88:260–7.
Cortes JE, Talpaz M, Giles F, O'Brien S, Rios MB, Shan J, et al. Prognostic significance of cytogenetic clonal evolution in patients with chronic myelogenous leukemia on imatinib mesylate therapy. Blood. 2003;101:3794–800.
Spiers ASD. Metamorphosis of chronic granulocytic leukemia: diagnosis, classification and management. Brit J Haematol. 1979;41:1–7.
Hehlmann R. How I treat CML blast crisis. Blood. 2012;120:737–47.
Cortes JE, Talpaz M, O'Brien S, Faderl S, Garcia-Manero G, Ferrajoli A, et al. Staging of chronic myeloid leukemia in the imatinib era: an evaluation of the World Health Organization proposal. Cancer. 2006;106:1306–15.
Hehlmann R, Hochhaus A, Baccarani M. Chronic myeloid leukemia. The Lancet. 2007;370:342–50.
Saußele S, Lauseker M, Gratwohl A, Beelen DW, Bunjes D, Schwerdtfeger R, et al. Allogeneic hematopoietic stem cell transplantation (alloSCT) for chronic myeloid leukemia in the imatinib era: evaluation of its impact within a subgroup of the randomized German CML Sudy IV. Blood. 2010;115:1880–5.
Gratwohl A, Pfirrmann M, Zander A, Kröger N, Beelen D, Novotny J, et al. Long-term outcome of patients with newly diagnosed chronic myeloid leukemia: a randomized comparison of stem cell transplantation with drug treatment. Leukemia. 2016;30:562–9.
Perrotti D, Jamieson C, Goldman J, Skorski T. Chronic myeloid leukemia: mechanisms of blastic transformation. J Clin Invest. 2010;120:2254–64.
Koptyra M, Falinski R, Nowicki MO, Stoklosa T, Majsterek I, Nieborowska-Skorska M, et al. BCR/ABL kinase induces self-mutagenesis via reactive oxygen species to encode imatinib resistance. Blood. 2006;108:319–27.
Fabarius A, Leitner A, Hochhaus A, Müller MC, Hanfstein B, Haferlach C, et al. Impact of additional cytogenetic aberrations at diagnosis on prognosis of CML: long-term observation of 1151 patients from the randomized CML Study IV. Blood. 2011;118:6760–8.
Wang W, Cortes JE, Tang G, Khoury JD, Wang S, Bueso-Ramos CE, et al. Risk stratification of chromosomal abnormalities in chronic myelogenous leukemia in the era of tyrosine kinase inhibitor therapy. Blood. 2016;127:2742–50.
Wang W, Cortes JE, Lin P, Beaty MW, Ai D, Amin HM, et al. Clinical and prognostic significance of 3q26.2 and other chromosome 3 abnormalities in CML in the era of tyrosine kinase inhibitors. Blood. 2015a;126:1699–706.
Wang W, Tang G, Cortes JE, Liu H, Ai D, Yin CC, et al. Chromosomal rearrangement involving 11q23 locus in chronic myelogenous leukemia: a rare phenomenon frequently associated with disease progression and poor prognosis. Journal of hematology & oncology. 2015b;8:32.
Wang W, Cortes JE, Lin P, Khoury JD, Ai D, Tang Z, et al. Impact of trisomy 8 on treatment response and survival of patients with CML in the era of tyrosine kinase inhibitors. Leukemia. 2015c;29:2263–6.
Gong Z, Medeiros LJ, Cortes JE, Chen Z, Zheng L, Li Y, et al. Cytogenetics-based risk prediction of blastic transformation of chronic myeloid leukemia in the era of TKI therapy. Blood Adv. 2017;1:2541–52.
Hehlmann R. Prognostic relevance of CCAs/Ph- in CML settled. Blood. 2017;130:2046–7.
Issa GC, Kantarjian H, Nogueras Gonzalez G, Borthakur G, Tang G, Wierda W, et al. Clonal chromosomal abnormalities appearing in Philadelphia chromosome-negative metaphases during CML treatment. Blood. 2017;130:2084–91.
Hehlmann R, Lauseker M, Saußele S, Pfirrmann M, Krause S, Kolb HJ, et al. Assessment of imatinib as first-line treatment of chronic myeloid leukemia: 10-year survival results of the randomized CML study IV and impact of non-CML determinants. Leukemia. 2017;31:2398–406.
Pfirrmann M, Baccarani M, Saussele S, Guilhot J, Cervantes F, Ossenkoppele G, et al. Prognosis of long-term survival considering disease-specific death in patients with chronic myeloid leukemia. Leukemia. 2016;30:48–56.
Fabarius A, Kalmanti L, Dietz CT, Lauseker M, Rinaldetti S, Haferlach C, et al. Impact of unbalanced minor route versus major route karyotypes at diagnosis on prognosis of CML. Ann Hematol. 2015;94:2015–24.
Hehlmann R, Müller MC, Lauseker M, Hanfstein B, Fabarius A, Schreiber A, et al. Deep molecular response is reached by the majority of patients treated with imatinib, predicts survival, and is achieved more quickly by optimized high-dose imatinib: results from the randomized CML-study IV. J Clin Oncol. 2014;32:415–23.
Cross NCP, White HE, Müller MC, Saglio G, Hochhaus A. Standardized definitions of molecular response in chronic myeloid leukemia. Leukemia. 2012;26:2172–5.
Cross NC, Hochhaus A, Müller MC. Molecular monitoring of chronic myeloid leukemia: principles and interlaboratory standardization. Ann Hematol. 2015;94(Suppl 2):S219–25.
Cross NC, White HE, Colomer D, Ehrencrona H, Foroni L, Gottardi E, et al. Laboratory recommendations for scoring deep molecular responses following treatment for chronic myeloid leukemia. Leukemia. 2015;29:999–1003.
Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872–84.
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision of the WHO classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–405.
Rea D, Etienne G, Nicolini F, Cony-Makhoul P, Johnson-Ansah H, Legros L, et al. First-line imatinib mesylate in patients with newly diagnosed accelerated phase-chronic myeloid leukemia. Leukemia. 2012;26:2254–9.
Branford S, Wang P, Yeung DT, Thomsen D, Purins A, Wadham C, et al. Integrative genomic analysis reveals cancer-associated mutations at diagnosis of CML in patients with high-risk disease. Blood. 2018;132:948–61.
Boullion A-S, Ventura Ferreira MS, Adnan Awad S, Richter J, Hochhaus A, Kunzmann V, et al. Telomere shortening correlates with leukemic stem cell burden at diagnosis of chronic myeloid leukemia. Blood Adv. 2018;2:1572–9.
Haaß W, Kleiner H, Weiß C, Haferlach C, Schlegelberger B, Müller MC, et al. Clonal evolution and blast crisis correlate with enhanced proteolytic activity of separase in BCR-ABL b3a2 fusion type CML under imatinib therapy. PLoS ONE. 2015. https://doi.org/10.1371/journal.pone.0129648:1-18.
Acknowledgements
We thank E Matzat, R Pleil-Lösch, I Stalljann, G Bartsch, C Sodan-Boyer, A Elett, M Meckesheimer, U Böhm, and J Hehlmann for assistance.
The SAKK and the German CML Study Group
L. Fischer von Weikersthal17, M. Hahn18, G. Schlimok19, D. Reichert20, J. Janssen20, U. Martens21, P. Majunke22, Peter Reichert23, K. Neben24, S. Korsten25, Ch. Scholz26, B. Oldenkott27, J. Heßling28, D. Kingreen29, C. Sperling30, C. Schelenz30, I. Blau31, A. Urmersbach32, W. Ludwig33, P. Le Coutre34, R. Arnold34, M. de Wit35, A. Pezzutto36, E. Schäfer37, R. Schroers38, A. Lochter38, D. Behringer39, Y. Ko40, S. Weidenhöfer40, W. Verbeek41, P. Brossart42, G. Trenn43, W. Pommerien44, J. Krauter45, G. Doering46, H. Munzinger46, C. Diekmann47, B. Hertenstein48, S. Stier49, F. Möller-Faßbender50, M. Hänel51, T. Zöller52, C. Lamberti53, B. Koch54, A. Henzel55, S. Wagner56, A. Schmalenbach57, M. Hoffknecht58, G. Ehninger59, A. Kiani59, T. Illmer60, C. Aul61, M. Flaßhove62, F. Henneke62, M. Simon63, L. Müller64, H. Becker64, R. Janz65, M. J. Eckart66, R. Fuchs67, F. Schlegel67, M. Wattad68, R. Rudolph69, D. W. Beelen70, A. Lindemann71, D. Linck72, Wassman73, E. Jäger74, S. Al-Batran74, T. Reiber75, C. F. Waller76, H. Hoeffkes77, L. Schulz78, K. Tajrobehkar79, J. Mittermüller80, H. Pralle81, V. Runde82, A. Hoyer83, H. Tessen83, L. Trümper84, C. Schmidt85, M. Sieber86, H. Eschenburg87, R. Depenbusch88, S. Rösel88, H. W. Lindemann89, H. Wolf90, C. Spohn91, R. Moeller91, D. Hossfeld92, A. Zander92, P. Schafhausen92, H. Köster93, W. Hollburg94, N. Schmitz95, H. Dürk96, M. Hemeier96, A. Grote-Metke97, H. Weischer97, B. Bechtel97, L. Balleisen98, M. Sosada99, A. Ho100, V. Petersen101, J. Dengler102, S. Bildat103, L. Hahn104, H. Dietzfelbinger105, W. Gröschel106, A. Bartholomäus107, W. Freier108, B. Sievers108, I.-M. Pfreundschuh109, T. Herrmann110, A. Fauser110, J. Menzel111, M. Kemmerling112, R. Hansen113, H. Link114, M. Schatz115, M. Bentz116, O. Prümmer117, M. Kneba118, J. Heymanns119, S. Schmitz120, C. Scheid121, A. Lollert122, M. Neise122, M. Planker123, M. Stauch124, M. Schröder125, B. Kempf126, U. Vehling-Kaiser127, S. Kremers128, G. Köchling129, L. Müller130, F. Hartmann131, T. Neuhaus132, S. Fetscher133, D. Kämpfe134, G. Heil135, M. Uppenkamp136, B. Goldmann137, T. Fischer Huber138, U. Hieber139, C. Plöger140, M. Griesshammer141, C. Lange142, B. Göttler143, C. Lunscken144, X. Schiel145, C. Scheidegger146, O. Stötzer147, H. Hitz148, H. Schick149, S. Völkl150, K. Spiekermann151, W. Berdel152, H. Hebart153, E. Ladda154, P. Schmidt155, U. Burkhardt155, S. Hentschke156, C. Falge157, D. Reschke158, C. A. Köhne159, C. Müller-Naendrup160, M. Sauer161, S. Frühauf162, K. Ranft163, Y. Dencausse164, B. Sandritter165, G. Baake166, M. Hofknecht167, R. Dengler168, M. Edinger169, M. Schenk170, A. Wehmeier171, H.-P. Weidelich172, R. Pihusch173, K. Stahlhut174, M. Baldus175, A. Matzdorff176, T. Geer177, S. Schanz178, G. Käfer179, W. Gassmann180, C. Priebe-Richter181, M. Demandt182, G. Springer183, H. Fiechtner183, C. Denzlinger184, J. Schleicher185, D. Assman186, R. Gaeckler187, G. Adam188, A. Waladkhani189, B. Rendenbach190, H. Forstbauer191, L. Kanz192, S. Jacki193, F. Stegelmann193, N. Kalhori194, A. Nusch194, W. Langer194, F. Müller195, S. Brettner196, B. Uebelmesser197, T. Kamp198, C. Schadeck-Gressel199, K. Josten200, O. Klein200, R. Schwerdtfeger201, H. Baurmann201, H. Strotkötter202, W. Fett203, A. Raghavachar204, C. Maintz205, M. C. Goebler206, R. Schlag207, W. Elsel208, M. Wernli209, D. Heim210, W. Wuillemin211, U. Hess212, J. Gmür213, J. Mayer214.
Funding
This work was funded by European LeukemiaNet (ELN) Foundation.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Members of the SAKK and the German CML Study Group are listed below Acknowledgements.
Appendix A1. Karyotypes of patients with ACA
Appendix A1. Karyotypes of patients with ACA
No. | Sex | Agea | Intervalb | Karyotype |
|---|---|---|---|---|
High-risk ACA in Ph+ cells | ||||
1 | F | 54 | 11 | 46,XX,inv(3)(q21q26),t(7;9;22)(q31;q34;q11) [20] |
16 | 46,XX,inv(3)(q21q26),t(7;9;22)(q31;q34;q11) [25] | |||
20 | 46,XX,inv(3)(q21q26),t(7;9;22)(q31;q34;q11) [25] | |||
2 | F | 45 | 28 | 46,XX,inv(3)(q21q26),t(9;22)(q34;q11) [19] |
32 | 46,XX,inv(3)(q21q26),t(9;22)(q34;q11) [2] | |||
3 | F | 61 | 14 | 46,XX,t(9;22)(q34;q11) [3]/46,XX,der(7)del(7)(p11)del(7)(q11),t(9;22)(q34;q11) [2]/ 45,XX,der(3;9;17)der(7)del(7)(p11)del(7)(q11),+der(9)ins(9;17)(p11;??),t(9;22)(q34;q11),der(9;17)dic(9;17) (q11;p11)del(9)(p13p24),der(14)t(1;14)(q21;q21),+17,der(22)t(9;22)(q34;q11) [2]/45,X,r(X),der(3;9;17) (3pter?....)der(7)del(7)(p11)del(7)(q11),+der(9)ins(9;17)(p11;??),t(9;22)(q34;q11),der(9;17)dic(9;17)(q11;p11)del(9)(p13p24),der(14)(q21;q21),+17,der(22)t(9;22)(q34;q11) [4] |
4 | M | 42 | 0 | 46,XY,inv(3)(p13q25),t(9;22)(q34;q11) [17] |
5 | F | 32 | 12 | 46,XX,inv(3)(q21q26),t(9;22)(q34;q11) [15] |
6 | M | 43 | 8 | 46,XY,inv(3)(q21q26),t(9;22)(q34;q11) [24] |
7 | F | 37 | 6 | 46,XX,t(3;21)(q26;q22),t(9;22)(q34;q11) [15] |
7 | 46,XX,t(3;21)(q26;q22),t(9;22)(q34;q11) [17] | |||
8 | 46,XX,t(3;21)(q26;q22),t(9;22)(q34;q11) [20] | |||
8 | M | 37 | 11 | 46,XY,inv(3)(q21q26),t(9;22)(q34;q11) [9] |
14 | 46,XY,inv(3)(q21q26),t(9;22)(q34;q11) [3] | |||
17 | 46,XY,inv(3)(q21q26),t(9;22)(q34;q11) [2] | |||
24 | 46,XY,inv(3)(q21q26),t(9;22)(q34;q11) [3] | |||
32 | 46,XY,inv(3)(q21q26),t(9;22)(q34;q11) [2] | |||
36 | 46,XY,inv(3)(q21q26),t(9;22)(q34;q11) [2] | |||
44 | 46,XY,inv(3)(q21q26),t(9;22)(q34;q11) [6] | |||
45 | 46,XY,inv(3)(q21q26),t(9;22)(q34;q11) [20] | |||
9 | M | 60 | 6 | 48–49,XY,t(5;9)(q31;q22),−7,der(7)del(7)(p11)del(7)(q11),+8, der(9)t(7;9)(q11;p23)t(9;22)(q34;q11),+19,der(22)t(9;22)(q34;q11),+der(22)t(9;22)[cp12] |
10 | M | 71 | 12 | 46,XY,der(5)t(5;9)(q11;?),−7,der(9)t(9;22)(q34;q11)t(9;9)(p13;?)t(7;9)(?;?), der(9)del(9)(p22)t(9;7)(q?;?)t(5;7)(q?;?),der(22)t(9;22)(q34;q11),+der(22)t(9;22)(q34;q11) [6] |
11 | M | 45 | 13 | 46,XY,r(7)(p11q32)del(7)(q11q22),del(9)(p12p24),t(9;22)(q34;q11) [6] |
12 | M | 36 | 25 | 43–44,XY,−8,t(9;22)(q34;q11),−11,−15,−17,−18,−19,−20,−22,+2–3mar[cp10] |
13 | M | 44 | 3 | 46,XY,t(9;22)(q34;q11) [11]/51,XY,+X,dup(6)(p22p25),+der(6)t(4;6)(q24;q16),t(9;22)(q34;q11),+14,+21, +der(22)t(9;22)(q34;q11) [4]/51,XY,+X,der(4)t(4;8)(p15;q22),dup(6)(p22p25),+der(6)t(4;6)(q24;q16),+14,+21, +der(22)t(9;22)(q34;q11) [2] |
14 | F | 68 | 10 | 46,XX,t(9;22)(q34;q11) [6]/46,XX,inv(3)(q21q26),t(9;22)(q34;q11) [6] |
13 | 46,XX,inv(3)(q21q26),t(9;22)(q34;q11) [2]/45,XX,inv(3)(q21q26),−7,t(9;22)(q34;q11) [10] | |||
15 | M | 69 | 9 | 46,XY,t(9;22)(q34;q11)/48,XY,+8,t(9;22)(q34;q11),+der(22)t(9;22)(q34;q11) [3]/ 50,XY+8,+8,t(9;22)(q34;q11),+19,+der(22)t(9;22)(q34;q11) [4] |
19 | 45,XY,t(3;12)(q26;q24),−7,t(9;22)(q34;q11) [11] | |||
16 | F | 54 | 45 | 46,XX,t(3;21)(q26;q22),t(9;22)(q34;q11) [25] |
48 | 46,XX,t(3;21)(q26;q22),t(9;22)(q34;q11) [25] | |||
17 | M | 64 | 22 | 51,XY,+6,+8,+8,der(9)t(9;10)(q34;q22),der(10)t(10;15)(q22;q15), +der(11)t(11;17)(p11.2;q11.2),der(15)t(15;22)(q15;q11.2),der(22)t(9;22)(q34;q11.2),+der(22)t(9;22)(q34;q11.2) [17] |
18 | M | 24 | 10 | 45,X,−Y,t(9;22)(q34;q11) [8]/46,X,−Y,t(9;22)(q34;q11.2),+der(22)t(9;22)(q34;q11.2) [4]/ 47,X,−Y,−7,t(9 ;22)(q34;q11.2),+der(22)t(9;22)(q34;q11.2),+mar,+r [8] |
19 | M | 61 | 0 | 46,XY,t(9;22)(q34;q11) [14]/46,XY,der(9)t(9;22)(q34;q11),ider(22)(q10)t(9;22)(q34;q11) [11] |
20 | M | 51 | 44 | 47,XY,t(9;22)(q34;q11),+der(22)t(9;22)(q34;q11) [4] |
21 | M | 61 | 46 | 46,XY,t(9;22)(q34;q11),+der(22)idic(9)(q34)t(9;22)(q34;q11),+der(22)t(9;22)(q34;q11)x3,+mar[cp20] |
64 | 49,XY,t(9;22)(q34;q11),+der(22)t(9;22)(q34;q11)x3 [3] | |||
22 | F | 60 | 2 | 46,XX,der(7)t(7;22)(q36;q11)del(7)(q11q22),der(9)t(7;9)(q36;q34),der(22)t(9;22)(q34;q11) [21] |
23 | M | 46 | 0 | 47,XY,der(9)t(9;22)(q34;q11),del(9)(q33q34),del(22)(q11q12),+der(22)t(9;22)(q34;q11) [25] |
24 | F | 59 | 6 | 46,XX,der(9)t(9;22;15)(q34;q11;q26),der(15)t(9;22;15),+22,der(22)idic(22)(p11)t(9;22) [15] |
25 | M | 34 | 3 | 48,XY,+X,der(5)t(1;5)(q21;q31),t(9;22;21)(q34;q11;q22),+der(22)t(9;22) [15] |
26 | M | 40 | 41 | 47,XY,t(9;22)(q34;q11),+der(22)t(9;22)(q34;q11) [2] |
76 | 47,XY,t(9;22)(q34;q11),+der(22)t(9;22)(q34;q11) [5] | |||
78 | 47,XY,t(9;22)(q34;q11),+der(22)t(9;22)(q34;q11) [8] | |||
79 | 47,XY,t(9;22)(q34;q11),+der(22)t(9;22)(q34;q11) [5] | |||
27 | M | 36 | 72 | 46,XY,t(9;22)(q34;q11)/47,XY,t(9;22)(q34;q11),+der(22)t(9;22)(q34;q11) [3] |
28 | M | 54 | 9 | 47,XY,t(9;22)(q34;q11),+der(22)t(9;22)(q34;q11) [6] |
29 | M | 44 | 29 | 46,XY,t(9;22)(q34;q11) [5]/47,XY,t(9;22)(q34;q11),+der(22)t(9;22)(q34;q11) [11] |
33 | 46,XY,t(9;22)(q34;q11) [3]/47,XY,t(9;22)(q34;q11),+der(22)t(9;22)(q34;q11) [9] | |||
30 | M | 32 | 18 | 47,XY,t(1;14)(p3?1;q?32),del(9)(p22p24),t(9;22)(q34;q11),+der(22)t(9;22)(q34;q11) [9]/ 46,XY,del(9)(p22p24),t(9;22)(q34;q11),del(12)(p11.2p13) [6] |
31 | M | 78 | 13 | 45,X,−Y,del(9)(q22),+der(22)t(9;22)(q34;q11)t(9;9)(q34;q22) [13] |
32 | M | 38 | 27 | 47,XY,t(9;22)(q34;q11),+der(22)t(9;22)(q34;q11) [14] |
33 | M | 23 | 0 | 46,XY,t(9;22)(q34;q11) [18]/47,idem,+der(22)t(9;22)(q34;q11) [2] |
34 | M | 68 | 19 | 46,XX,t(9;22)(q34;q11) [5]/48,XX,t(9;22)(q34;q11),+19,+der(22)t(9;22)(q34;q11) [8] |
35 | F | 69 | 0 | 46,XX,t(9;22)(q34;q11) [8]/47,idem,+ider(22)(q10)t(9;22)(q34;q11) [7] |
3 | 46,XX,t(9;22)(q34;q11) [7]/47,idem,ider(22)(q10)t(9;22)(q34;q11) [3] | |||
36 | M | 51 | 27 | 46,XY,t(9;22)(q34;q11) [14]/48,idem,+19,+der(22)t(9;22)(q34;q11) [2] |
37 | F | 60 | 35 | 45,XX,−7,t(9;22)(q34;q11) [7] |
41 | 46,XX,r(7)(p13q11),t(9;22)(q34;q11) [9]/45,XX,−7,t(9;22)(q34;q11) [4] | |||
44 | 46,XX,r(7)(p13q11),t(9;22)(q34;q11) [7]/45,XX,−7,t(9;22)(q34;q11) [2] | |||
38 | M | 39 | 12 | 46,XY,t(9;22)(q34;q11) [7]/47,XY,+8,t(9;22)(q34;q11) [2]/48,XY,+8,t(9;22)(q34;q11),+der(22)t(9;22)(q34;q11) [3] |
88 | 46,XY,t(9;22)(q34;q11) /47,XY,+8,t(9;22)(q34;q11) /48,XY,+8,t(9;22)(q34;q11),+der(22)t(9;22)(q34;q11) [16] | |||
39 | F | 58 | 7 | 55,XX,+X,+5,+6,+7,+8,t(9;22)(q34;q11),+14,+19,+22,+der(22)t(9;22)(q34;q11)[cp5] |
40 | M | 38 | 15 | 47,XY,t(9;22)(q34;q11),+8 [2] |
41 | M | 39 | 0 | 47,XY,+8,t(9;22)(q34;q11) [2]/47,XY,+8,t(9;22)(q34;q11),i(17)(q10) [2]/48,XY,+8,t(9;22)(q34;q11),i(17)(q10),+22, del(22)q11) [10] |
42 | M | 64 | 10 | 46,XY,t(9;22)(q34;q11) [16]/50,XY,+8,+12,+18,+21,t(9;22)(q34;q11) [3] |
43 | M | 34 | 5 | |
117 | ||||
44 | F | 54 | 73 | |
81 | 47,XX,+der(8),t(1;8)(q22;p22),t(9;22)(q34;q11) [2] | |||
45 | F | 42 | 51 | |
46 | M | 72 | 0 | 47,XY,+8,t(9;22)(q34;q11) [20] |
7 | ||||
47 | M | 40 | 0 | 47,XY,+8,t(9;22)(q34;q11) [17]/49,XY,+8,t(9;22)(q34;q11),+19,+20 [7] |
48 | M | 25 | 0 | |
49 | F | 24 | 23 | |
29 | ||||
36 | ||||
40 | 47,XX,+8,t(9;22)(q34;q11) [20] | |||
44 | 47,XX,+8,t(9;22)(q34;q11) [5] | |||
50 | M | 31 | 0 | 46,XY,t(9;22;10)(q34;q11;p15) [13]/55,XY,+3,+8,t(9;22;10)(q34;q11;p15),+12,+13,+14,+18,+19,+21,+der(22) t(9;22(q34;q11) [7] |
51 | M | 28 | 0 | 46,XY,t(9;22)(q34;q11) [10]/47,XY,t(9;22)(q34;q11),+der(22)t(9;22)(q34:q11) [10] |
3 | 46,XY,t(9;22)(q34;q11) /47,XY,t(9;22)(q34;q11),+der(22)t(9;22)(q34:q11) [20] | |||
52 | M | 55 | 0 | 46,XY,t(9;22)(q34;q11) [7]/49,XY,+8,+10,t(9;22)(q34;q11),+der(22)t(9;22)(q34:q11) [13] |
6 | 46,XY,t(9;22)(q34;q11) [3]/49,XY,+8,+10,t(9;22)(q34;q11),+der(22)t(9;22)(q34:q11) [12] | |||
53 | F | 68 | 0 | 48,XX,+8,t(9;18)(q34;q21),+der(22)ins(22;9)(q11;34q34) [5] |
6 | 48,XX,+8,t(9;18)(q34;q21),+der(22)ins(22;9)(q11;34q34) [4] | |||
54 | M | 38 | 0 | 47,XY,+8,t(9;22)(q34;q11) [3]/48,XY,+8,t(9;22)(q34;q11),+der(22)t(9;22)(q34;q11) [11] |
55 | M | 53 | 0 | 50,XY,+8,+8,i(17)(q10),+19,+der(22)t(9;22)(q34;q11) [6] |
56 | M | 28 | 10 | 45,XY,t(8;21)(q22;q22),t(9;22)(q34;q11),−11/93,XXYY,+8,+8,t(8;21)(q22;q22),t(9;22)(q34;q11),−11 [8] |
57 | M | 70 | 19 | |
64 | ||||
58 | M | 55 | 0 | 46,XY,t(1;12)(p34;q24),t(1;9;22)(p36;q34;q11) [21]/48,idem,+8,+9 [2] |
59 | M | 46 | 52 | |
72 | ||||
80 | 47,XY,+8,t(9;22)(q34;q11) [4] | |||
86 | ||||
60 | M | 64 | 42 | |
53 | ||||
57 | ||||
65 | ||||
69 | ||||
61 | M | 57 | 0 | 48,XY,+8,t(9;22)(q34;q11),+19 [25] |
62 | M | 50 | 76 | 46,XY,t(9;22)(q34;q11)/47,XY,+8,t(9;22)(q34;q11),idic(17)(p11) [24] |
63 | M | 46 | 102 | 47,XY,+8,t(9;22)(q34;q11),i(17)(q10) [6] |
64 | M | 41 | 0 | 46,XY,t(9;22)(q34;q11) [10]/44,XY,t(9;22)(q34;q11),−14,i(17)(q10),−18 [15] |
65 | F | 62 | 10 | 46,XX,t(1;9;22)(p36;q34;q11) [8]/47,XX,t(1;9;22)(p36;q34;q11),+8 [2] |
66 | F | 76 | 8 | 47,XX,+8,t(9;22)(q34;q11) [3] |
67 | M | 44 | 20 | |
34 | 49,XY,t(3;21)(q26;q22),+8,t(9;22)(q34;q11),+12,+der(22)t(9;22)(q34;q11) [4]/46,XY [19] | |||
68 | F | 52 | 0 | 47,XX,+8,t(9;22)(q34;q11),i(17)(q10) [10] |
3 | ||||
8 | 50,XX,+8,+8,+8,t(9;22)(q34;q11),idic(17)(p12),+19 [7]/46,XX [20] | |||
69 | F | 56 | 0 | |
70 | M | 48 | 0 | |
71 | M | 51 | 0 | 46,XY,t(9;22)((q34;q11) [7]/47,XY,+8,t(9;22)(q34;q11),i(17)(q10) [18] |
72 | F | 75 | 24 | 47,XX,t(9;22)(q34;q11),+21 [4] |
73 | F | 39 | 0 | 46,XX,t(9;22)(q34;q11) [3]/46,idem,t(20;21)(q10;q10) /47,idem,t(20;21)(q10;q10),+21 [11] |
74 | M | 42 | 19 | 47,XY,t(9;22)(q34;q11),+17 [20] |
21 | 47,XY,t(9;22)(q34;q11),+17 [10] | |||
75 | F | 62 | 11 | 46,XX,t(9;11)(p21–22;q23),t(9;22)(q34;q11) [25] |
76 | M | 51 | 38 | 46,XY,t(9;22)(q34;q11),inv(16)(p13q22),+der(22)t(9;22)(q34;q11) [3] |
77 | F | 59 | 32 | 48,XX,t(9;15;22) (q34;q26;q11),+8,+19 [3] |
78 | F | 51 | 7 | 45,XX,der(7;9)(q10;q10)t(9;22)(q34;q11) [8] |
79 | M | 24 | 8 | 46,XY,der(9)t(9;22)(q34;q11),der(19)t(9;19)(q34;p13);der(22)t(9;22)(q34;q11) t(9;19)(q34,p13) [2] |
80 | M | 53 | 0 | 46,XY,der(9)t(9;22)(q34;q11),idicder(22)(q11)t(9;22)(q34;q11), idicder(22)(q11)t(9;22)(q34;q11) [3] |
81 | M | 55 | 0 | 46,XY,der(7)t(7;9)(q11.2;q34),der(9)t(9;22)(q34;q11.2),der(22)t(9;22)(q34;q11.2) t(7;9)(q11.2q34) [13] |
82 | M | 40 | 0 | 47,XY,+8,t(9;22)(q34;q11) [16] |
83 | F | 68 | 13 | 46,XX,t(3;11)(q26;q23),t(9;22)(q34;q11) [20] |
84 | F | 54 | 45 | 46,XX,t(3;21)(q26;q22),t (9;22)(q34;q11) [25] |
85 | 42 | 40 | 47,XY,der(8)t(8;9)(p21;p13),+der(8)t(8;9)(p21;p13),del(9)(p13p24), der(9)t(8;9)(p21;p11)t(9;10)(q34;q24),der(10)t(10;22)(q24;q11), t(14;21)(q22;q21),der(22)t(9;22)(q34;q11) [10] | |
86 | M | 60 | 22 | 46,XY,inv(7)(p22q32),+8,t(9;22)(q34;q11) [20] |
87 | M | 44 | 0 | 46,XY,der(9)t(9;10)(q22;q23)t(10;22)(q25;q11),der(10)t(9;10)(q22;q23)t(9;15)(q34;q21),der(15)t(10;15)(q26;q15) t(10;22)(q25;q11),der(22)t(9;22)(q34;q11) [21] |
4 | 46,XY,der(9)t(9;10)(q22;q23)t(10;22)(q25;q11),der(10)t(9;10)(q22;q23)t(9;15)(q34;q21),der(15)t(10;15)(q26;q15) t(10;22)(q25;q11),der(22)t(9;22)(q34;q11) [8] | |||
7 | 46,XY,der(9)t(9;10)(q22;q23)t(10;22)(q25;q11),der(10)t(9;10)(q22;q23)t(9;15)(q34;q21),der(15)t(10;15)(q26;q15) t(10;22)(q25;q11),der(22)t(9;22)(q34;q11) [3] | |||
10 | 46,XY,der(9)t(9;10)(q22;q23)t(10;22)(q25;q11),der(10)t(9;10)(q22;q23)t(9;15)(q34;q21),der(15)t(10;15)(q26;q15) t(10;22)(q25;q11),der(22)t(9;22)(q34;q11) [3] | |||
88 | F | 38 | 0 | 46,XX,del(1)(q21),der(9)t(9;22)(q34;q11)t(1;22)(q44;q11),der(22)t(9;22)(q34;q11)t(1;9)(q21;q34) [20] |
89 | F | 66 | 0 | 46,XX,del(1)(q32),der(9)t(1;9)(q32;q34)t(1;22)(q44;q11),der(22)t(9;22)(q34;q11) [20] |
90 | M | 55 | 0 | 46,XY,t(7;11),del(7q),der(9)t(9;22)(q34;q11),der(11),del(16q),der(17),der(22)t(9;22)(q34;q11) [10] |
91 | F | 47 | 0 | 46,XX,t(9;22)(q34;q11) [2]/46,XX,der(7;11)ins(7;11)(p14;p11q25)t(7;11)(p22;p11),t(9;22)(q34;q11) [13] |
3 | 46,XX,t(9;22)(q34;q11) [5]/45,XX,der(7;11)ins(7;11)(p14;p11q25)t(7;11)(p22;p11),t(9;22)(q34;q11) [14]/ 46,XX,t(9;22)(q34;q11),del(11)(p13) [2]/46,XX,del(4)(q31),der(11)t(4;11)(q?;p13)t(11;13)(q21;q14), der(12)t(11;12)(p13;q24),der(13)t(11;13)(q21;q14) [2] | |||
6 | 46,XX,t(9;22)(q34;q11) [2]/45,XX,der(7;11)ins(7;11)(p14;p11q25)t(7;11)(p22;p11),t(9;22)(q34;q11) [9] | |||
Low-risk ACA in Ph+ cells | ||||
92 | M | 59 | 0 | 46,XY,t(9;22)(q34;q11),del(15)(q22),add(17)(p11) [8] |
4 | 46,XY,t(9;22)(q34;q11),del(15)(q22),add(17)(p11) [7] | |||
7 | 46,XY,t(9;22)(q34;q11),del(15)(q22),add(17)(p11) [10] | |||
10 | 46,XY,t(9;22)(q34;q11),del(15)(q22),add(17)(p11) [7] | |||
93 | M | 48 | 0 | 46,XY,t(4;6)(q21;p23),t(9;22)(q34;q11) [20] |
94 | M | 36 | 12 | 46,XY,t(9;22)(q34;q11) [8]/46,XY,t(9;22)(q34;q11),i(9)(p10),der(17)t(9;17)(q11;p11) [12] |
95 | F | 58 | 0 | |
96 | M | 44 | 0 | 46,XY,der(10),t(9;22)(q34;q11) [25] |
97 | M | 46 | 0 | 46,XY,t(9;22)(q34;q11),t(14;17)(p11;q11) [20] |
98 | M | 40 | 0 | 46,XY,t(9;22)(q34;q11) [2]/46,XY,del(5)(q11q14),t(9;22)(q34;q11) [19] |
6 | 46,XY,del(5)(q11q14),t(9;22)(q34;q11) [2] | |||
99 | F | 51 | 7 | 46,XX,t(9;22)(q34;q11) [4]/45,XX,der(7;9)(q10;q10)t(9;22)(q34;q11),der(22)t(9;22)(q34;q11) [8] |
100 | M | 27 | 0 | |
101 | F | 52 | 0 | 46,XX,t(2;16)(p2?3;p1?3),t(9;22)(q34;q11) [26] |
102 | F | 61 | 0 | 46,XX,t(9;22)(q34;q11) [21]/46,XX,del(6)(q15q23),t(9;22)(q34;q11) [4] |
103 | F | 68 | 0 | 46,XX,t(9;22)(q34;q11) /46,XX,del(5)(q13q22),t(9;22)(q34;q11) [24] |
104 | F | 64 | 0 | 46,XX,t(5;8)(q14;q23),t(9;22)(q34;q11) [19] |
105 | M | 37 | 0 | 46,XY,t(9;22)(q34;q11),t(15;20)(q13;p12) [20] |
106 | F | 45 | 39 | 46,XX,t(9;22)(q34;q11.2) [16]/46,XX,t(9;22)(q34;q11.2),add(20)(p11.2) [9] |
107 | M | 19 | 0 | 46,XY,der(1)t(1;9)(q21;q34)t(9;22)(q34:q11),der(9)t(1;9)(q21;q34)t(9;22)(q34:q11),der(22)t(9;22)(q34;q11) [3] |
108 | F | 65 | 86 | 46,XX,t(9;22)(q34;q11) [17]/46,XX,del(X)(p?21),t(9;22)(q34;q11) [6] |
109 | M | 64 | 10 | 46,XY,add(9)(q34),add(9)(q32–34),der(22)t(9;22)(q34;q11) [10] |
14 | 46,XY,t(1;3)(p36;q2?6),add(9)(q34),add(9)(q32–34),der(22)t(9;22)(q34;q11) [10] | |||
110 | F | 56 | 0 | 46,XX,t(1;21)(q21;q22),t(9;22)(q34;q11) [20] |
12 | 46,XX,t(1;21)(q21;q22),t(9;22)(q34;q11) [15] | |||
24 | 46,XX,t(1;21)(q21;q22),t(9;22)(q34;q11) [2] | |||
111 | M | 36 | 0 | 46,XY,t(9;22)(q34;q11),t(11;19)(q14.1;q13) [20] |
112 | M | 62 | 0 | 46,XY,t(9;22)(q34;q11) [2]/46,idem,add(8)(q24) [2]/45,idem,der(18)t(10;18)(q11;p11) [10] |
113 | M | 61 | 0 | 46,XY,t(1;9)(q24;q31),t(9;22)(q34;q11) [20] |
114 | F | 46 | 0 | 46,XX,der(2)t(2;4)(q37;q21)del(4)(q21),t(9;22)(q34;q11) [20] |
115 | M | 70 | 0 | 46,XY,der(9)t(9;22)(q34;q11),t(10;22)(q25;q13) [17] |
4 | 46,XY,der(9)t(9;22)(q34;q11),t(10;22)(q25;q13) [3] | |||
7 | 45,X,−Y [3]/46,XY,der(9)t(9;22)(q34;q11),t(10;22)(q25;q13) [2] | |||
116 | F | 69 | 94 | 46,XX,t(9;22)(q34;q11),?del(17)(p12) [4] |
117 | F | 49 | 0 | 46,XX,del(3)(p11p2?1)or(p21),t(9;22)(q34;q11) [12]/46,XX,del(3)(p11p2?1)or(p21),del(5)(q15q31),t(9;22)(q34;q11) /46,XX,t(9;22)(q34;q11) [2] |
118 | F | 76 | 0 | 46,XX,t(9;22)(q34;q11) [7]/46,XX,t(9;22)(q34;q11),der(19)t(19;?)(p13.3;?) [13] |
4 | 46,XX,t(9;22)(q34;q11) [7]/46,XX,t(9;22)(q34;q11),der(19)t(17;19)(q22;p13) [13] | |||
119 | M | 40 | 3 | 46,XY,t(6;15),t(9;22)(q34;q11) [6] |
120 | M | 66 | 11 | 46,XY,t(9;22)(q34;q11) [9]/46,XY,der(6)t(6;17)(p21;q11),t(9;22)(q34;q11) [11] |
121 | F | 43 | 0 | 46,XX,t(7;7)(p22;q22),t(9;22;9)(q34;q11;p24) [16] |
60 | 40–43,XX,t(7;7)(p22;q22),t(9;22;9)(q34;q11;p24),inc [cp3] | |||
122 | M | 24 | 6 | 46,XY,t(2;12)(q33;p13),t(9;22)(q34;q11) [20] |
123 | F | 31 | 11 | 46,XX,t(9;22)(q34;q11) [18]/ 46,XX,t(9;22)(q34;q11),ins(11;11)(p15;p11.2p13) [4] |
25 | 46,XX,t(9;22)(q34;q11) [9]/ 46,XX,t(9;22)(q34;q11),ins(11;11)(p15;p11.2p13) [5] | |||
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hehlmann, R., Voskanyan, A., Lauseker, M. et al. High-risk additional chromosomal abnormalities at low blast counts herald death by CML. Leukemia 34, 2074–2086 (2020). https://doi.org/10.1038/s41375-020-0826-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-020-0826-9
This article is cited by
-
Cytogenetic and epidemiological profile of chronic myeloid leukemia in Morocco
Annals of Hematology (2024)
-
Management and outcome of patients with chronic myeloid leukemia in blast phase in the tyrosine kinase inhibitor era – analysis of the European LeukemiaNet Blast Phase Registry
Leukemia (2024)
-
Survival with chronic myeloid leukaemia after failing milestones
Leukemia (2023)
-
Pathogenesis and management of accelerated and blast phases of chronic myeloid leukemia
Leukemia (2023)
-
Management of children and adolescents with chronic myeloid leukemia in blast phase: International pediatric CML expert panel recommendations
Leukemia (2023)