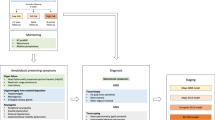
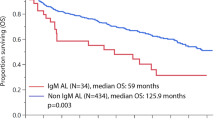
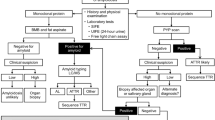
Abstract
Systemic amyloidosis is a rare but increasingly recognised disease that is heterogeneous in presentation. Early diagnosis, whilst imperative, remains challenging but can improve prognosis and limit organ dysfunction. An increased repertoire of diagnostic imaging and histological techniques are becoming mainstream and promise to aid early diagnosis. Better risk stratification, via biomarkers and cytogenetics, has improved multidisciplinary treatment decisions. The use of novel agents has improved treatment efficacy, which translates into survival benefit. Newer strategies targeting pre-deposited amyloidogenic protein are under investigation. The current paper reviews available data relating to the most recent advances in the field of systemic amyloidosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med. 2003;349:583–96.
Quock TP, Yan T, Chang E, Guthrie S, Broder MS. Epidemiology of AL amyloidosis: a real-world study using US claims data. Blood Adv. 2018;2:1046–53.
Lousada I, Comenzo RL, Landau H, Guthrie S, Merlini G. Light chain amyloidosis: patient experience survey from the amyloidosis research consortium. Adv Ther. 2015;32:920–8.
Weiss BM, Hebreo J, Cordaro DV, Roschewski MJ, Baker TP, Abbott KC, et al. Increased serum free light chains precede the presentation of immunoglobulin light chain amyloidosis. J Clin Oncol. 2014;32:2699–704.
Gonzalez-Lopez E, Gallego-Delgado M, Guzzo-Merello G, de Haro-Del Moral FJ, Cobo-Marcos M, Robles C, et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J. 2015;36:2585–94.
Quarta CC, Gonzalez-Lopez E, Gilbertson JA, Botcher N, Rowczenio D, Petrie A, et al. Diagnostic sensitivity of abdominal fat aspiration in cardiac amyloidosis. Eur Heart J. 2017;38:1905–8.
Vrana JA, Gamez JD, Madden BJ, Theis JD, Bergen HR III, Dogan A. Classification of amyloidosis by laser microdissection and mass spectrometry-based proteomic analysis in clinical biopsy specimens. Blood. 2009;114:4957–9.
Winter M, Tholey A, Kristen A, Rocken C. MALDI mass spectrometry imaging: a novel tool for the identification and classification of amyloidosis. Proteomics. 2017;17:1700236.
Kotecha T, Martinez-Naharro A, Treibel TA, Francis R, Nordin S, Abdel-Gadir A, et al. Myocardial edema and prognosis in amyloidosis. J Am Coll Cardiol. 2018;71:2919–31.
Banypersad SM, Fontana M, Maestrini V, Sado DM, Captur G, Petrie A, et al. T1 mapping and survival in systemic light-chain amyloidosis. Eur Heart J. 2015;36:244–51.
Martinez-Naharro A, Abdel-Gadir A, Treibel TA, Zumbo G, Knight DS, Rosmini S, et al. Regression of cardiac al amyloid by cardiovascular magnetic resonance. Circulation. 2016;134:A14407.
Gillmore JD, Maurer MS, Falk RH, Merlini G, Damy T, Dispenzieri A, et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation. 2016;133:2404–12.
Quarta CC, Obici L, Guidalotti PL, Pieroni M, Longhi S, Perlini S, et al. High 99mTc-DPD myocardial uptake in a patient with apolipoprotein AI-related amyloidotic cardiomyopathy. Amyloid. 2013;20:48–51.
Hawkins PN. Serum amyloid P component scintigraphy for diagnosis and monitoring amyloidosis. Curr Opin Nephrol Hypertens. 2002;11:649–55.
Manwani R, Page J, Lane T, Burniston M, Skillen A, Lachmann HJ, et al. A pilot study demonstrating cardiac uptake with 18F-florbetapir PET in AL amyloidosis patients with cardiac involvement. Amyloid. 2018;25:247–52.
Ezawa N, Katoh N, Oguchi K, Yoshinaga T, Yazaki M, Sekijima Y. Visualization of multiple organ amyloid involvement in systemic amyloidosis using (11)C-PiB PET imaging. Eur J Nucl Med Mol Imaging. 2018;45:452–61.
Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Colby C, et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol. 2012;30:989–95.
Merlini G, Lousada I, Ando Y, Dispenzieri A, Gertz MA, Grogan M, et al. Rationale, application and clinical qualification for NT-proBNP as a surrogate end point in pivotal clinical trials in patients with AL amyloidosis. Leukemia. 2016;30:1979–86.
Muchtar E, Dispenzieri A, Leung N, Lacy MQ, Buadi FK, Dingli D, et al. Depth of organ response in AL amyloidosis is associated with improved survival: grading the organ response criteria. Leukemia. 2018;32:2240–9.
Palladini G, Barassi A, Perlini S, Milani P, Foli A, Russo P, et al. Midregional proadrenomedullin (MR-proADM) is a powerful predictor of early death in AL amyloidosis. Amyloid. 2011;18:216–21.
Kastritis E, Papassotiriou I, Merlini G, Milani P, Terpos E, Basset M, et al. Growth differentiation factor-15 is a new biomarker for survival and renal outcomes in light chain amyloidosis. Blood. 2018;131:1568–75.
Kastritis E, Gavriatopoulou M, Dimopoulos MA, Eleutherakis-Papaiakovou E, Kanellias N, Roussou M, et al. Osteoprotegerin is a significant prognostic factor for overall survival in patients with primary systemic amyloidosis independent of the Mayo staging. Blood Cancer J. 2015;5:e319.
Kastritis E, Papassotiriou I, Terpos E, Roussou M, Gavriatopoulou M, Komitopoulou A, et al. Clinical and prognostic significance of serum levels of von Willebrand factor and ADAMTS-13 antigens in AL amyloidosis. Blood. 2016;128:405–9.
Dispenzieri A, Gertz MA, Saenger A, Kumar SK, Lacy MQ, Buadi FK, et al. Soluble suppression of tumorigenicity 2 (sST2), but not galactin-3, adds to prognostication in patients with systemic AL amyloidosis independent of NT-proBNP and troponin T. Am J Hematol. 2015;90:524–8.
Abraham J, Desport E, Rigaud C, Marin B, Bender S, Lacombe C, et al. Hepatocyte growth factor measurement in AL amyloidosis. Amyloid. 2015;22:112–6.
Kristen AV, Rosenberg M, Lindenmaier D, Merkle C, Steen H, Andre F, et al. Osteopontin: a novel predictor of survival in patients with systemic light-chain amyloidosis. Amyloid. 2014;21:202–10.
Sachchithanantham S, Berlanga O, Alvi A, Mahmood SA, Lachmann HJ, Gillmore JD, et al. Immunoparesis defined by heavy+light chain suppression is a novel marker of long-term outcomes in cardiac AL amyloidosis. Br J Haematol. 2017;179:575–85.
Bhole MV, Sadler R, Ramasamy K. Serum-free light-chain assay: clinical utility and limitations. Ann Clin Biochem. 2014;51:528–42.
Milani P, Murray DL, Barnidge DR, Kohlhagen MC, Mills JR, Merlini G, et al. The utility of MASS-FIX to detect and monitor monoclonal proteins in the clinic. Am J Hematol. 2017;92:772–9.
Sharpley FA, Manwani R, Mahmood S, Sachchithanantham S, Lachmann HJ, Gillmore JD, et al. A novel mass spectrometry method to identify the serum monoclonal light chain component in systemic light chain amyloidosis. Blood Cancer J. 2019;9:16.
Kourelis TV, Kumar SK, Gertz MA, Lacy MQ, Buadi FK, Hayman SR, et al. Coexistent multiple myeloma or increased bone marrow plasma cells define equally high-risk populations in patients with immunoglobulin light chain amyloidosis. J Clin Oncol. 2013;31:4319–24.
Sidana S, Tandon N, Dispenzieri A, Gertz MA, Dingli D, Jevremovic D, et al. Prognostic significance of circulating plasma cells by multi-parametric flow cytometry in light chain amyloidosis. Leukemia. 2018;32:1421–6.
Muchtar E, Dispenzieri A, Kumar SK, Ketterling RP, Dingli D, Lacy MQ, et al. Interphase fluorescence in situ hybridization in untreated AL amyloidosis has an independent prognostic impact by abnormality type and treatment category. Leukemia. 2017;31:1562–9.
Bochtler T, Hegenbart U, Kunz C, Benner A, Kimmich C, Seckinger A, et al. Prognostic impact of cytogenetic aberrations in AL amyloidosis patients after high-dose melphalan: a long-term follow-up study. Blood. 2016;128:594–602.
Bochtler T, Hegenbart U, Kunz C, Benner A, Seckinger A, Dietrich S, et al. Gain of chromosome 1q21 is an independent adverse prognostic factor in light chain amyloidosis patients treated with melphalan/dexamethasone. Amyloid. 2014;21:9–17.
Walker BA, Rowczienio D, Boyle EM, Wardell CP, Sachchithanantham S, Baginska A, et al. Exome sequencing to define a genetic signature of plasma cells in systemic al amyloidosis. Blood. 2013;122:3098.
Huang XF, Jian S, Lu JL, Shen KN, Feng J, Zhang CL, et al. Genomic profiling in amyloid light-chain amyloidosis reveals mutation profiles associated with overall survival. Amyloid. 2020;27:36–44.
da Silva Filho MI, Forsti A, Weinhold N, Meziane I, Campo C, Huhn S, et al. Genome-wide association study of immunoglobulin light chain amyloidosis in three patient cohorts: comparison with myeloma. Leukemia. 2017;31:1735–42.
Manwani R, Foard D, Mahmood S, Sachchithanantham S, Lane T, Quarta C, et al. Rapid hematologic responses improve outcomes in patients with very advanced (stage IIIb) cardiac immunoglobulin light chain amyloidosis. Haematologica. 2018;103:e165–8.
Muchtar E, Dispenzieri A, Leung N, Lacy MQ, Buadi FK, Dingli D, et al. Optimizing deep response assessment for AL amyloidosis using involved free light chain level at end of therapy: failure of the serum free light chain ratio. Leukemia. 2019;33:527–31.
Manwani R, Sharpley F, Mahmood S, Sachchithanantham S, Lachmann H, Gillmore J, et al. Achieving a difference in involved and uninvolved light chains (dFLC) of less than 10mg/L is the new goal of therapy in systemic AL amyloidosis: analysis of 916 patients treated upfront with bortezomib-based therapy. Blood. 2018;132:3262.
Muchtar E, Jevremovic D, Dispenzieri A, Dingli D, Buadi FK, Lacy MQ, et al. The prognostic value of multiparametric flow cytometry in AL amyloidosis at diagnosis and at the end of first-line treatment. Blood. 2017;129:82–7.
Sidana S, Tandon N, Dispenzieri A, Gertz MA, Rajkumar SV, Kumar SK. The importance of bone marrow examination in patients with light chain amyloidosis achieving a complete response. Leukemia. 2018;32:1243–6.
Palladini GMM, Basset M, Russo F, Milani P, Foli A, Merlini G. Persistence of minimal residual disease by multiparameter flow cytometry can hinder recovery of organ damage in patients with AL amyloidosis otherwise in complete response. Blood. 2016;128:3261.
D’Souza A, Dispenzieri A, Wirk B, Zhang MJ, Huang J, Gertz MA, et al. Improved outcomes after autologous hematopoietic cell transplantation for light chain amyloidosis: a center for international blood and marrow transplant research study. J Clin Oncol. 2015;33:3741–9.
Sharpley F, Petrie, A, Mahmood, S, Sachchithanantham, S, Lachmann, HJ, Gillmore, JD, et al. A twenty-four year experience of autologous stem cell transplantation for light chain amyloidosis patients in the United Kingdom. Br J Haematol. 2019;187:642–52.
Sanchorawala V, Sun FG, Quillen K, Sloan JM, Berk JL, Seldin DC. Long-term outcome of patients with AL amyloidosis treated with high-dose melphalan and stem cell transplantation: 20-year experience. Blood. 2015;126:2345–7.
Sanchorawala V, Brauneis D, Shelton AC, Lo S, Sun FG, Sloan JM, et al. Induction therapy with bortezomib followed by bortezomib-high dose melphalan and stem cell transplantation for light chain annyloidosis: results of a prospective clinical trial. Biol Blood Marrow Transplant. 2015;21:1445–51.
Manwani R, Hegenbart U, Mahmood S, Sachchithanantham S, Kyriakou C, Yong K, et al. Deferred autologous stem cell transplantation in systemic AL amyloidosis. Blood Cancer J. 2018;8:101.
Wong SW, Larivee D, Warner M, Sprague KA, Fogaren T, Comenzo RL. Stem cell transplantation in patients with systemic AL amyloidosis referred for transplant after suboptimal responses to bortezomib-based initial therapy. Bone Marrow Transplant. 2017;52:936–7.
Landau H, Smith M, Landry C, Chou JF, Devlin SM, Hassoun H, et al. Long-term event-free and overall survival after risk-adapted melphalan and SCT for systemic light chain amyloidosis. Leukemia. 2017;31:136–42.
Kastritis E, Leleu X, Arnulf B, Zamagni E, Cibeira MT, Kwok F, et al. A randomized phase III trial of melphalan and dexamethasone (MDex) versus bortezomib, melphalan and dexamethasone (BMDex) for untreated patients with AL amyloidosis. Blood. 2016;128:646.
Palladini G, Sachchithanantham S, Milani P, Gillmore J, Foli A, Lachmann H, et al. A European collaborative study of cyclophosphamide, bortezomib, and dexamethasone in upfront treatment of systemic AL amyloidosis. Blood. 2015;126:612–5.
Manwani R, Cohen O, Sharpley F, Mahmood S, Sachchithanantham S, Foard D, et al. A prospective observational study of 915 patients with systemic AL amyloidosis treated with upfront bortezomib. Blood. 2019;134:2271–80.
Kastritis E, Gavriatopoulou M, Roussou M, Fotiou D, Ziogas DC, Migkou M, et al. Addition of cyclophosphamide and higher doses of dexamethasone do not improve outcomes of patients with AL amyloidosis treated with bortezomib. Blood Cancer J. 2017;7:e570.
Dispenzieri A, Lacy MQ, Zeldenrust SR, Hayman SR, Kumar SK, Geyer SM, et al. The activity of lenalidomide with or without dexamethasone in patients with primary systemic amyloidosis. Blood. 2007;109:465–70.
Sanchorawala V, Wright DG, Rosenzweig M, Finn KT, Fennessey S, Zeldis JB, et al. Lenalidomide and dexamethasone in the treatment of AL amyloidosis: results of a phase 2 trial. Blood. 2007;109:492–6.
Kastritis E, Gavriatopoulou M, Roussou M, Bagratuni T, Migkou M, Fotiou D, et al. Efficacy of lenalidomide as salvage therapy for patients with AL amyloidosis. Amyloid. 2018;25:234–41.
Dinner S, Witteles W, Afghahi A, Witteles R, Arai S, Lafayette R, et al. Lenalidomide, melphalan and dexamethasone in a population of patients with immunoglobulin light chain amyloidosis with high rates of advanced cardiac involvement. Haematologica. 2013;98:1593–9.
Hegenbart U, Bochtler T, Benner A, Becker N, Kimmich C, Kristen AV, et al. Lenalidomide/melphalan/dexamethasone in newly diagnosed patients with immunoglobulin light chain amyloidosis: results of a prospective phase 2 study with long-term follow up. Haematologica. 2017;102:1424–31.
Dispenzieri A, Buadi F, Laumann K, LaPlant B, Hayman SR, Kumar SK, et al. Activity of pomalidomide in patients with immunoglobulin light-chain amyloidosis. Blood. 2012;119:5397–404.
Sanchorawala V, Shelton AC, Lo S, Varga C, Sloan JM, Seldin DC. Pomalidomide and dexamethasone in the treatment of AL amyloidosis: results of a phase 1 and 2 trial. Blood. 2016;128:1059–62.
Palladini G, Milani P, Foli A, Basset M, Russo F, Perlini S, et al. A phase 2 trial of pomalidomide and dexamethasone rescue treatment in patients with AL amyloidosis. Blood. 2017;129:2120–3.
Sharpley FA, Manwani R, Mahmood S, Sachchithanantham S, Lachmann H, Gilmore J, et al. Real world outcomes of pomalidomide for treatment of relapsed light chain amyloidosis. Br J Haematol. 2018;183:557–63.
Shaulov A, Ganzel C, Benyamini N, Barshay Y, Goldschmidt N, Lavie D, et al. Progressive refractory light chain amyloidosis and multiple myeloma patients are responsive to the addition of clarithromycin to IMiD based therapy. Am J Hematol. 2017;92:131–5.
Puig NHM, Rosinol Dachs L, Gonzalez Garcia E, De Arriba F, Oriol A, Gonzalez-Calle V, et al. Randomized trial of lenalidomide and dexamethasone versus clarythromycin, lenalidomide and dexamethasone as first line treatment in patients with multiple myeloma not candidates for autologous stem cell transplantation: results of the GEM-Claridex clinical trial. Orlando, USA: American Society of Haematology; 2019.
Cohen AD, Landau H, Scott EC, Liedtke M, Kaufman JL, Rosenzweig M, et al. Safety and efficacy of carfilzomib (CFZ) in previously-treated systemic light-chain (AL) amyloidosis. Blood. 2016;128:645.
Garg MHA, Jenner M, Kishore B, Lachmann HJ, Gillmore JD, Pitchford A, et al. A phase 1 study of carfilzomib-thalidomide-dexamethasone in patients with relapsed/refractory AL amyloidosis—catalyst trial results. Orlando, USA: American Society of Haematology Conference; 2019.
Sanchorawala V, Palladini G, Kukreti V, Zonder JA, Cohen AD, Seldin DC, et al. A phase 1/2 study of the oral proteasome inhibitor ixazomib in relapsed or refractory AL amyloidosis. Blood. 2017;130:597–605.
Dispenzieri AKE, Wechalekar AD, Schonland SO, Kim K, Sanchorawala V, Landau HJ, et al. Primary results from the phase 3 tourmaline-AL1 trial of ixazomib-dexamethasone versus physician’s choice of therapy in patients (Pts) with relapsed/refractory primary systemic AL amyloidosis (RRAL). Orlando, FL, USA: American Society of Haematology 2019.
Kaufman GP, Schrier SL, Lafayette RA, Arai S, Witteles RM, Liedtke M. Daratumumab yields rapid and deep hematologic responses in patients with heavily pretreated AL amyloidosis. Blood. 2017;130:900–2.
Abeykoon JP, Zanwar S, Dispenzieri A, Gertz MA, Leung N, Kourelis T, et al. Daratumumab-based therapy in patients with heavily-pretreated AL amyloidosis. Leukemia. 2019;33:531–6.
Comenzo RL, Kastritis E, Maurer M, Zonder J, Minnema MC, Wechalekar A, et al. Subcutaneous daratumumab + cyclophosphamide, bortezomib, and dexamethasone (cybord) in patients with newly diagnosed amyloid light chain (AL) amyloidosis: updated safety run-in results of andromeda. Amsterdam: European Haematology Assocation; 2019.
Sidiqi MH, Saleh AS Al, Leung N, Alijama M, Jevremovic D, Gonslaves W, et al. Venetoclax for the treatment of translocation (11; 14) Al amyloidosis. Amsterdam: European Haematology Association; 2019.
Premkumar V, Comenzo R, Lentzsch S. Venetoclax in immunoglobulin light chain amyloidosis: is this the beginning or the end? Clin Lymphoma Myeloma Leuk. 2019;19:686–8.
Le Bras FDJ, Lemonnier F, Oghina S, Bodez S, Ladaique A, Maarek A, et al. Venetoclax induces sustained complete responses in refractory/relapsed patients with cardiac AL amyloidosis. J Clin Oncol. 2019;37:e19538.
Zago W, Renz M, Torres R, Dolan PJ, Barbour RM, Salmans JR, et al. NEOD001 specifically binds aggregated light chain infiltrates in multiple organs from patients with AL amyloidosis and promotes phagocytic clearance of AL aggregates in vitro. Blood. 2015;126:3016.
Gibbs SDJ, De Cruz M, Sattianayagam PT, Lachmann HJ, Gillmore JD, Hawkins PN, et al. Transient post chemotherapy rise in NT Pro-BNP in AL amyloidosis: implications for organ response assessment. Blood. 2009;114:712.
Gertz MACA, Comenzo RL, Du Mond C, Kastritis E, Landau HJ, Libby III EL, et al. Results of the phase 3 VITAL study of NEOD001 (Birtamimab) plus standard of care in patients with light chain (AL) amyloidosis suggest survival benefit for mayo stage IV patients. Orlando, FL, USA: American Society of Haematology; 2019.
Richards DB, Cookson LM, Barton SV, Liefaard L, Lane T, Hutt DF, et al. Repeat doses of antibody to serum amyloid P component clear amyloid deposits in patients with systemic amyloidosis. Sci Transl Med. 2018;10:3128.
Edwards CV, Gould J, Langer AL, Mapara M, Radhakrishnan J, Maurer MS, et al. Interim analysis of the phase 1a/b study of chimeric fibril-reactive monoclonal antibody 11-1F4 in patients with AL amyloidosis. Amyloid. 2017;24:58–9.
Swuec P, Lavatelli F, Tasaki M, Paissoni C, Rognoni P, Maritan M, et al. Cryo-EM structure of cardiac amyloid fibrils from an immunoglobulin light chain AL amyloidosis patient. Nat Commun. 2019;10:1269.
Radamaker L, Lin YH, Annamalai K, Huhn S, Hegenbart U, Schonland SO, et al. Cryo-EM structure of a light chain-derived amyloid fibril from a patient with systemic AL amyloidosis. Nat Commun. 2019;10:1103.
Wechalekar AD, Whelan C. Encouraging impact of doxycycline on early mortality in cardiac light chain (AL) amyloidosis. Blood Cancer J. 2017;7:e546.
Ericzon BG, Wilczek HE, Larsson M, Wijayatunga P, Stangou A, Pena JR, et al. Liver transplantation for hereditary transthyretin amyloidosis: after 20 years still the best therapeutic alternative? Transplantation. 2015;99:1847–54.
Berk JL, Suhr OB, Obici L, Sekijima Y, Zeldenrust SR, Yamashita T, et al. Repurposing diflunisal for familial amyloid polyneuropathy: a randomized clinical trial. JAMA. 2013;310:2658–67.
Merkies IS. Tafamidis for transthyretin familial amyloid polyneuropathy: a randomized, controlled trial. Neurology. 2013;80:1444–5.
Adams D, Gonzalez-Duarte A, O’Riordan WD, Yang CC, Ueda M, Kristen AV, et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med. 2018;379:11–21.
Benson MD, Waddington-Cruz M, Berk JL, Polydefkis M, Dyck PJ, Wang AK, et al. Inotersen treatment for patients with hereditary transthyretin amyloidosis. N Engl J Med. 2018;379:22–31.
Solomon SD, Adams D, Kristen A, Grogan M, Gonzalez-Duarte A, Maurer MS, et al. Effects of patisiran, an RNA interference therapeutic, on cardiac parameters in patients with hereditary transthyretin-mediated amyloidosis. Circulation. 2019;139:431–43.
Morgan GJ, Yan NL, Mortenson DE, Rennella E, Blundon JM, Gwin RM, et al. Stabilization of amyloidogenic immunoglobulin light chains by small molecules. Proc Natl Acad Sci USA. 2019;116:8360–9.
Hovey BM, Ward JE, Soo Hoo P, O’Hara CJ, Connors LH, Seldin DC. Preclinical development of siRNA therapeutics for AL amyloidosis. Gene Ther. 2011;18:1150–6.
Wechalekar AD, Gillmore JD, Hawkins PN. Systemic amyloidosis. Lancet. 2016;387:2641–54.
Kaku M, Berk JL. Neuropathy associated with systemic amyloidosis. Semin Neurol. 2019;39:578–88.
Westermark GT, Fandrich M, Westermark P. AA amyloidosis: pathogenesis and targeted therapy. Annu Rev Pathol. 2015;10:321–44.
Sethi S, Theis JD. Pathology and diagnosis of renal non-AL amyloidosis. J Nephrol. 2018;31:343–50.
Sidiqi MH, Aljama MA, Buadi FK, Warsame RM, Lacy MQ, Dispenzieri A, et al. Stem cell transplantation for light chain amyloidosis: decreased early mortality over time. J Clin Oncol. 2018;36:1323–9.
Palladini G, Milani P, Foli A, Vidus Rosin M, Basset M, Lavatelli F, et al. Melphalan and dexamethasone with or without bortezomib in newly diagnosed AL amyloidosis: a matched case-control study on 174 patients. Leukemia. 2014;28:2311–6.
Wechalekar AD, Goodman HJB, Lachmann HJ, Offer M, Hawkins PN, Gillmore JD. Safety and efficacy of risk-adapted cyclophosphamide, thalidomide, and dexamethasone in systemic AL amyloidosis. Blood. 2007;109:457–64.
Mahmood S, Venner CP, Sachchithanantham S, Lane T, Rannigan L, Foard D, et al. Lenalidomide and dexamethasone for systemic AL amyloidosis following prior treatment with thalidomide or bortezomib regimens. Br J Haematol. 2014;166:842–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cohen, O.C., Wechalekar, A.D. Systemic amyloidosis: moving into the spotlight. Leukemia 34, 1215–1228 (2020). https://doi.org/10.1038/s41375-020-0802-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-020-0802-4
This article is cited by
-
Minimal residual disease negativity by next-generation flow cytometry is associated with improved organ response in AL amyloidosis
Blood Cancer Journal (2021)
-
Redirecting proteoxicity
Leukemia (2020)