Abstract
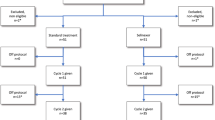
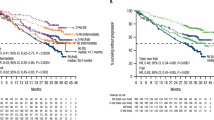
More effective treatment modalities are urgently needed in patients with acute myeloid leukemia (AML) of older age. We hypothesized that adding lenalidomide to intensive standard chemotherapy might improve their outcome. After establishing a safe lenalidomide, dose elderly patients with AML were randomly assigned in this randomized Phase 2 study (n = 222) to receive standard chemotherapy (“3 + 7”) with or without lenalidomide at a dose of 20 mg/day 1–21. In the second cycle, patients received cytarabine 1000 mg/m2 twice daily on days 1–6 with or without lenalidomide (20 mg/day 1–21). The CR/CRi rates in the two arms were not different (69 vs. 66%). Event-free survival (EFS) at 36 months was 19% for the standard arm versus 21% for the lenalidomide arm and overall survival (OS) 35% vs. 30%, respectively. The frequencies and grade of adverse events were not significantly different between the treatment arms. Cardiovascular toxicities were rare and equally distributed between the arms. The results of the present study show that the addition of lenalidomide to standard remission induction chemotherapy does not improve the therapeutic outcome of older AML patients. This trial is registered as number NTR2294 in The NederlandsTrial Register (www.trialregister.nl).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
30 July 2020
A Correction to this paper has been published: https://doi.org/10.1038/s41375-020-0994-7
References
Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373:1136–52.
Ossenkoppele G, Löwenberg B. How I treat the older patient with acute myeloid leukemia. Blood. 2015;125:767–74.
Krug U, Gale RP, Berdel WE, Müller-Tidow C, Stelljes M, Metzeler K, et al. Therapy of older persons with acute myeloid leukaemia. Leuk Res. 2017;60:1–10.
Löwenberg B, Ossenkoppele GJ, van Putten W, Schouten HC, Graux C, Ferrant A. Dutch-Belgian Cooperative Trial Group for Hemato-Oncology (HOVON) et al. Therapy of older persons with acute myeloid leukaemia. Leuk Res. 2017;60:1–10.
Löwenberg B, Ossenkoppele GJ, van Putten W, Schouten HC, Graux C, Ferrant A. Swiss Group for Clinical Cancer Research (SAKK) Collaborative Group et al. High-dose daunorubicin in older patients with acute myeloid leukemia. N Engl J Med. 2009;361(Sep):1235–48.
Castaigne S, Pautas C, Terré C, Raffoux E, Bordessoule D, Bastie JN, Acute Leukemia French Association. et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet. 2012;379:1508–16.
Pigneux A, Béné MC, Salmi LR, Dumas PY, Delaunay J, Bonmati C, French Innovative Leukemia Organization, et al; Improved survival by adding lomustine to conventional chemotherapy for elderly patients with aml without unfavorable cytogenetics: results of the LAM-SA 2007 FILO trial. J Clin Oncol. 2018:JCO2018787366. https://doi.org/10.1200/JCO.2018.78.7366.
Lancet JE, Uy GL, Cortes JE, Newell LF, Lin TL, Ritchie EK, et al. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J Clin Oncol. 2018;36:2684–92.
Levin-Epstein R, Oliai C, Schiller G. Allogeneic hematopoietic stem cell transplantation for older patients with acute myeloid leukemia. Curr Treat Options Oncol. 2018;19:63.
Kotla V, Goel S, Nischal S, Heuck C, Vivek K, Das B, et al. Mechanism of action of lenalidomide in hematological malignancies. J Hematol Oncol. 2009;2:36 https://doi.org/10.1186/1756-8722-2-36
Fehniger TA, Uy GL, Trinkaus K, Nelson AD, Demland J, Abboud CN, et al. A phase 2 study of high-dose lenalidomide as initial therapy for older patients with acute myeloid leukemia. Blood. 2011;117:1828–33.
Zeidan AM, Smith BD, Carraway HE, Gojo I, DeZern A, Gore SD. A phase 2 trial of high dose lenalidomide in patients with relapsed/refractory higher-risk myelodysplastic syndromes and acute myeloid leukaemia with trilineage dysplasia. Br J Haematol. 2017;176:241–7.
Griffiths EA, Brady WE, Tan W, Vigil CE, Thompson JE, Ford LA, et al. A phase I study of intermediate dose cytarabine in combination with lenalidomide in relapsed/refractory acute myeloid leukemia. Leuk Res. 2016;43:44–8.
DeAngelo DJ, Brunner AM, Werner L, Avigan D, Fathi AT, Sperling AS, et al. A phase I study of lenalidomide plus chemotherapy with mitoxantrone, etoposide, and cytarabine for the reinduction of patients with acute myeloid leukemia. Am J Hematol. 2018;93:254–61.
Löwenberg B, Pabst T, Maertens J, van Norden Y, Biemond BJ, Schouten HC, Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON) and Swiss Group for Clinical Cancer Research (SAKK) et al. Therapeutic value of clofarabine in younger and middle-aged (18-65 years) adults with newly diagnosed AML. Blood. 2017;129:1636–45.
Terwijn M, van Putten WL, Kelder A, van der Velden VH, Brooimans RA, Pabst T, et al. High prognostic impact of flow cytometric minimal residual disease detection in acute myeloid leukemia: data from the HOVON/SAKK AML 42A study. J Clin Oncol. 2013;31:3889–9.
Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–47.
Stein EM, DiNardo CD, Pollyea DA, Fathi AT, Roboz GJ, Altman JK, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130:722–31.
DiNardo CD, Stein EM, de Botton S, Roboz GJ, Altman JK, Mims AS, et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med. 2018;378:2386–98.
Cortes J, Perl AE, Döhner H, Kantarjian H, Martinelli G, Kovacsovics T, et al. Quizartinib, an FLT3 inhibitor, as monotherapy in patients with relapsed or refractory acute myeloid leukaemia: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2018;19:889–903.
Wei AH, Strickland SA Jr, Hou JZ, Fiedler W, Lin TL, Walter RB, et al. Venetoclax combined with low-dose cytarabine for previously untreated patients with acute myeloid leukemia: results from a Phase Ib/II Study. J Clin Oncol. 2019:JCO1801600. https://doi.org/10.1200/JCO.18.01600.
DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133:7–17.
Beyar-Katz O, Gill S. Novel approaches to acute myeloid leukemia immunotherapy. Clin Cancer Res. 2018;24:5502–15.
Acknowledgements
The authors thanks the local and central data managers as well as the HOVON Datacenter Trial team. Dutch Cancer Foundation for financial support. Celgene for free drug supply and financial support to conduct the study.
The Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON) and Swiss Group for Clinical Cancer Research (SAKK)
D. A. Breems30, Havelange31, M-C Vekemans31, I. Moors32, F. van Obberg33, J. A. Maertens34, B. Hodossy35, S. Vansteenweghen35, L. Lammertijn35, D. Deeren36, C. Graux37, A. Sonet37, A. Triffet38, B. T. Gjertsen11, M. Bargetzi5, J. Passweg39, D. Heim39, San Giovanni40, Georg Stuessi40, T. Pabst28, D. Betticher41, Y. Chalandon42, O. Spertini43, M. Gregor44, U. Hess45, M. Fehr45, M. G. Manz46, S. K. Klein17, B. J. Biemond6, G J Ossenkoppele47, A. van de Loosdrecht47, J J W M Janssen47, J. W. J. van Esser48, M. Van der Klift48, R. E. Brouwer49, D. Van Lammeren-Venema50, M. D. Levin51, L. W. Tick52, M. C. J. C. Legdeur53, G. Huls54, E. Vellenga54, M. Hoogendoorn55, J. H. Veelken56, P. A. von dem Borne56, H. C. Schouten57, O. de Weerdt58, W. J. F. M. van der Velden59, J. Cornelissen15, M. Jongen-Lavrencic15, B. Wouters15, H. G. M. Raaijmakers15, B. Löwenberg15, J. Kuball60, A. Van Rhenen60, M. Van Marwijk Kooy61
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no relevant competing financial interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Members of the Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON) and Swiss Group for Clinical Cancer Research (SAKK) are listed below Acknowledgements
Supplementary information
Rights and permissions
About this article
Cite this article
Ossenkoppele, G.J., Breems, D.A., Stuessi, G. et al. Lenalidomide added to standard intensive treatment for older patients with AML and high-risk MDS. Leukemia 34, 1751–1759 (2020). https://doi.org/10.1038/s41375-020-0725-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-020-0725-0
This article is cited by
-
Age and sex associate with outcome in older AML and high risk MDS patients treated with 10-day decitabine
Blood Cancer Journal (2023)
-
Effects of lenalidomide on the bone marrow microenvironment in acute myeloid leukemia: Translational analysis of the HOVON103 AML/SAKK30/10 Swiss trial cohort
Annals of Hematology (2021)