Abstract
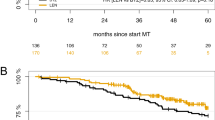
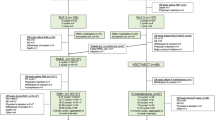
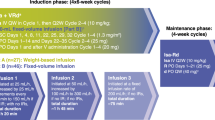
The MM5 trial aimed at demonstrating a progression-free survival (PFS) difference in continued vs. response-adapted (in case of complete response, CR) lenalidomide (LEN) maintenance therapy (MT) in newly diagnosed, transplant-eligible multiple myeloma (MM). Patients were equally randomized to receive induction therapy with PAd (bortezomib/doxorubicin/dexamethasone) or VCD (bortezomib/cyclophosphamide/dexamethasone), high-dose melphalan and autologous blood stem cell transplantation, and LEN consolidation, followed by either LEN MT for a fixed duration of 2 years (LEN-2Y) or until achievement of CR (LEN-CR, intention-to-treat population n = 502): arms A1:PAd + LEN-2Y (n = 125), B1:PAd + LEN-CR (n = 126), A2:VCD + LEN-2Y (n = 126), B2:VCD + LEN-CR (n = 125). In the LEN-CR group (B1 + B2), n = 88/17.5% patients did not start or discontinued LEN MT due to CR. There was no PFS (p = 0.60, primary endpoint) nor overall survival (OS) (p = 0.15) difference between the four study arms. On pooled LEN MT strategies, OS (hazard ratio, hazard ratio [HR] = 1.42, p = 0.03) but not PFS (HR = 1.15, p = 0.20) was shorter in LEN-CR (B1 + B2) vs. LEN-2Y (A1 + A2) groups. PFS was shortened on landmark analyses from the start of LEN MT in patients being in CR in the LEN-CR group (LEN-CR vs. LEN-2Y, HR = 1.84, p = 0.02). OS from first progression was shortened in the LEN-CR vs. LEN-2Y group (HR = 1.60, p = 0.01). LEN MT should be applied beyond CR for at least 2 years.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M, et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N. Engl J Med. 2017;376:1311–20.
Palumbo A, Cavallo F, Gay F, Di Raimondo F, Ben Yehuda D, Petrucci MT, et al. Autologous transplantation and maintenance therapy in multiple myeloma. N. Engl J Med. 2014;371:895–905.
Attal M, Lauwers-Cances V, Marit G, Caillot D, Moreau P, Facon T, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N. Engl J Med. 2012;366:1782–91.
Palumbo A, Hajek R, Delforge M, Kropff M, Petrucci MT, Catalano J, et al. Continuous lenalidomide treatment for newly diagnosed multiple myeloma. N. Engl J Med. 2012;366:1759–69.
McCarthy PL, Owzar K, Hofmeister CC, Hurd DD, Hassoun H, Richardson PG, et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N. Engl J Med. 2012;366:1770–81.
Jackson GH, Davies FE, Pawlyn C, Cairns DA, Striha A, Collett C, et al. Lenalidomide maintenance versus observation for patients with newly diagnosed multiple myeloma (Myeloma XI): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2019;20:57–73.
McCarthy PL, Holstein SA, Petrucci MT, Richardson PG, Hulin C, Tosi P, et al. Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: a meta-analysis. J Clin Oncol. 2017;35:3279–89.
Palumbo A, Bringhen S, Kumar SK, Lupparelli G, Usmani S, Waage A, et al. Second primary malignancies with lenalidomide therapy for newly diagnosed myeloma: a meta-analysis of individual patient data. Lancet Oncol. 2014;3:333–42.
Kumar SK, Dispenzieri A, Lacy MQ, Gertz MA, Buadi FK, Pandey S, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2013;5:1122–8.
Mai EK, Bertsch U, Dürig J, Kunz C, Haenel M, Blau IW, et al. Phase III trial of bortezomib, cyclophosphamide and dexamethasone (VCD) versus bortezomib, doxorubicin and dexamethasone (PAd) in newly diagnosed myeloma. Leukemia. 2015;8:1721–9.
Greipp PR, San Miguel J, Durie BGM, Crowley JJ, Barlogie B, Bladé J, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23:3412–20.
Neben K, Lokhorst HM, Jauch A, Bertsch U, Hielscher T, van der Holt B, et al. Administration of bortezomib before and after autologous stem cell transplantation improves outcome in multiple myeloma patients with deletion 17p. Blood. 2012;119:940–8.
Durie BGM, Harousseau J-L, Miguel JS, Bladé J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–73.
Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2008;23:3–9.
Marcus R, Eric P, Gabriel KR. On closed testing procedures with special reference to ordered analysis of variance. Biometrika. 1976;63:655–60.
Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–6.
Sonneveld P, Avet-Loiseau H, Lonial S, Usmani S, Siegel D, Anderson KC, et al. Treatment of multiple myeloma with high-risk cytogenetics: a consensus of the International Myeloma Working Group. Blood. 2016;127:2955–62.
Munshi NC, Anderson KC, Bergsagel PL, Shaughnessy J, Palumbo A, Durie B, et al. Consensus recommendations for risk stratification in multiple myeloma: report of the International Myeloma Workshop Consensus Panel 2. Blood. 2011;117:4696–4700.
Barlogie B, Crowley J. Could CR mean cure? Blood. 2011;118:483–483.
Paiva B, Gutiérrez NC, Rosiñol L, Vídriales M-B, Montalbán M-Á, Martínez-López J, et al. High-risk cytogenetics and persistent minimal residual disease by multiparameter flow cytometry predict unsustained complete response after autologous stem cell transplantation in multiple myeloma. Blood. 2012;119:687–91.
Martinez-Lopez J, Lahuerta JJ, Pepin F, González M, Barrio S, Ayala R, et al. Prognostic value of deep sequencing method for minimal residual disease detection in multiple myeloma. Blood. 2014;20:3073–9.
Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328–e346.
de Tute RM, Cairns D, Rawstron A, Pawlyn C, Davies FE, Jones JR, et al. Minimal residual disease in the maintenance setting in myeloma: prognostic significance and impact of lenalidomide. Blood. 2017;130:904–904.
Engelhardt M, Terpos E, Kleber M, Gay F, Wäsch R, Morgan G, et al. European Myeloma Network recommendations on the evaluation and treatment of newly diagnosed patients with multiple myeloma. Haematologica. 2014;99:232–42.
Gandolfi S, Prada CP, Richardson PG. How I treat the young patient with multiple myeloma. Blood. 2018;132:1114–24.
Moreau P, Attal M, Hulin C, Arnulf B, Belhadj K, Benboubker L, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet. 2019;394:29–38.
Acknowledgements
The GMMG-MM5 trial was supported by Celgene, Janssen-Cilag, Chugai, and The Binding Site. The GMMG thanks the Koordinierungszentrum für Klinische Studien (KKS) Heidelberg for the support of the trial and data monitoring. The GMMG thanks all participating investigators and centers (Supplementary Material 3).
Author information
Authors and Affiliations
Consortia
Contributions
Conception and design: HG, UB, CK and TH. Responsible statisticians: CK, TH and DT. Administrative support: HG, UB and SL. Provision of study materials or patients and/or collection, assembly and review of data: all authors. Data analysis and interpretation: HG, EKM, UB, MSR, TH, TD and CK. Writing of the first manuscript draft: EKM, HG, UB, CK and TH. Manuscript editing, discussion of trial data/results, and final writing: all authors. Final approval of manuscript: all authors.
Corresponding author
Ethics declarations
Conflict of interest
HG: honoraria: Amgen, BMS, Celgene, Chugai, Janssen, Novartis, Takeda; consulting or advisory role: Amgen, BMS, Celgene, Chugai, Janssen, Novartis, Takeda; speakers bureau: Amgen, BMS, Celgene, Janssen, Novartis, Takeda; research funding: Amgen, BMS, Celgene, Chugai, Janssen, Novartis, Takeda; travel, accomodations, expenses: BMS, Celgene, Janssen, Novartis, Takeda. EKM: honoraria: Janssen, Celgene, Takeda; consulting or advisory role: Janssen, Celgene, Takeda; research funding: Takeda; travel, accomodations, expenses: Janssen, Takeda, Celgene, Onyx, Mundipharma. JD: consulting or advisory role: Celgene; speakers bureau: Celgene; travel, accomodations, expenses: Celgene. CS: honoraria: BMS, Janssen, Celgene, Novartis, Amgen, Takeda; consulting or advisory role: BMS, Janssen, Celgene, Novartis, Amgen, Takeda; speakers bureau: Takeda; research funding: Takeda, Novartis; travel, accomodations, expenses: BMS, Janssen, Celgene, Novartis, Amgen, Takeda. KCW: honoraria: AMGEN, BMS, Celgene, Novartis, Janssen, Takeda; consulting or advisory role: AMGEN, BMS, Celgene, Juno, Janssen, Adaptive, Sanofi, Takeda; research funding: AMGEN, Celgene, Sanofi, Janssen; CK: no COI. UB: travel, accomodations, expenses: Sanofi TH: no COI. MMe: consulting or advisory role: Amgen, Takeda; research funding: Takeda; travel, accomodations, expenses: Celgen, AMGEN, Takeda, Abbvie, Janssen. MMu: honoraria: Janssen, BMS, Takeda, Celgene, Amgen; consulting or advisory role: Janssen, BMS, Takeda, Celgene, Amgen; research funding: BMS; travel, accomodations, expenses: Janssen, BMS, Takeda, Amgen. HWL: no COI. BHD: no COI. DT: no COI. NG: consulting or advisory role: Pfizer; travel, accomodations, expenses: Celgene, BMS. DH: consulting or advisory role: I. Lamkap Bio AG. Discoveric AG; research funding: Celgene AG. Sanofi. Engmab AG; travel, accomodations, expenses: Celgene. AS: consulting or advisory role: I. Lamkap Bio AG. Discoveric AG; research funding: Celgene AG. Sanofi. Engmab AG. SH: honoraria: Janssen; travel, accomodations, expenses: Celgene. SL: no COI. AJ: no COI. AE: consulting or advisory role: Amgen; travel, accomodations, expenses: Janssen, Amgen. BR: no COI. SF: consulting or advisory role: Sanofi, BMS, Amgen. PB: consulting or advisory role: BMS, AMGEN,Roche, MSD; research funding: BMS; travel, accomodations, expenses: BMS. MG: no COI. HB: no COI. MH: honoraria: MSD. JH: honoraria: AMGEN, Janssen; research funding: Celgene; travel, accomodations, expenses: AMGEN, Janssen. MSR: honoraria: Celgene, BMS, Novartis, Janssen, Takeda; consulting or advisory role: Celgene, BMS, Novartis, Janssen, Takeda; research funding: Celgene, Novartis, AMGEN; travel, accomodations, expenses: Janssen, BMS, Takeda. IWB: research funding: Celgene, BMS, Janssen. MH: honoraria: Novartis, Amgen, Roche, Takeda; consulting or advisory role: Celgene. HJS: honoraria: Celgene, Janssen, Cilag; travel, accomodations, expenses: Celgene, Janssen, Cilag.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Goldschmidt, H., Mai, E.K., Dürig, J. et al. Response-adapted lenalidomide maintenance in newly diagnosed myeloma: results from the phase III GMMG-MM5 trial. Leukemia 34, 1853–1865 (2020). https://doi.org/10.1038/s41375-020-0724-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-020-0724-1
This article is cited by
-
Artificial intelligence–based, volumetric assessment of the bone marrow metabolic activity in [18F]FDG PET/CT predicts survival in multiple myeloma
European Journal of Nuclear Medicine and Molecular Imaging (2024)
-
Predictors of early morbidity and mortality in newly diagnosed multiple myeloma: data from five randomized, controlled, phase III trials in 3700 patients
Leukemia (2024)
-
Implications and prognostic impact of mass spectrometry in patients with newly-diagnosed multiple myeloma
Blood Cancer Journal (2023)
-
Intensifying treatment in PET-positive multiple myeloma patients after upfront autologous stem cell transplantation
Leukemia (2023)
-
Impact of the changing landscape of induction therapy prior to autologous stem cell transplantation in 540 newly diagnosed myeloma patients: a retrospective real-world study
Journal of Cancer Research and Clinical Oncology (2023)