Abstract
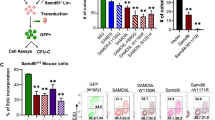
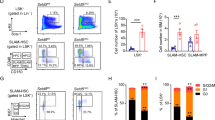
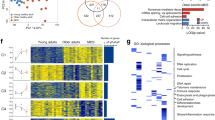
U2AF1 is involved in the recognition of the 3′ splice site during pre-mRNA splicing. Mutations in U2AF1 are frequently observed in myelodysplastic syndromes. However, the role of wild-type U2AF1 in normal hematopoiesis has remained elusive. Using a novel conditional U2af1 knockout allele, we have found that deletion of U2af1 results in profound defects in hematopoiesis characterized by pancytopenia, ablation of hematopoietic stem/progenitor cells (HSPC) leading to bone marrow failure and early lethality in mice. U2af1 deletion impairs HSPC function and repopulation capacity. U2af1 deletion also causes increased DNA damage and reduced survival in hematopoietic progenitors. RNA sequencing analysis reveals significant alterations in the expression of genes related to HSC maintenance, cell proliferation, and DNA damage response-related pathways in U2af1-deficient HSPC. U2af1 deficiency also induces splicing alterations in genes important for HSPC function. This includes altered splicing and perturbed expression of Nfya and Pbx1 transcription factors in U2af1-deficient HSPC. Collectively, these results suggest an important role for U2af1 in the maintenance and function of HSPC in normal hematopoiesis. A better understanding of the normal function of U2AF1 in hematopoiesis is important for development of appropriate therapeutic approaches for U2AF1 mutant induced hematologic malignancies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The RNA-Sequencing datasets generated in this study are deposited to NCBI GEO database (GSE162888).
References
Yoshida K, Sanada M, Shiraishi Y, Nowak D, Nagata Y, Yamamoto R, et al. Frequent Pathway Mutations of Splicing Machinery in Myelodysplasia. Nature. 2011;478:64–69.
Papaemmanuil E, Cazzola M, Boultwood J, Malcovati L, Vyas P, Bowen D, et al. Somatic SF3B1 Mutation in Myelodysplasia With Ring Sideroblasts. N Engl J Med. 2011;365:1384–95.
Graubert TA, Shen D, Ding L, Okeyo-Owuor T, Lunn CL, Shao J, et al. Recurrent mutations in the U2AF1 splicing factor in myelodysplastic syndromes. Nat Genet. 2012;44:53–57.
Thol F, Kade S, Schlarmann C, Löffeld P, Morgan M, Krauter J, et al. Frequency and Prognostic Impact of Mutations in SRSF2, U2AF1, and ZRSR2 in Patients With Myelodysplastic Syndromes. Blood. 2012;119:3578–84.
Damm F, Kosmider O, Gelsi-Boyer V, Renneville A, Carbuccia N, Hidalgo-Curtis C, et al. Groupe Francophone des Myélodysplasies., Mutations Affecting mRNA Splicing Define Distinct Clinical Phenotypes and Correlate With Patient Outcome in Myelodysplastic Syndromes. Blood. 2012;119:3211–18.
Ogawa S. Genetics of MDS. Blood. 2019;133:1049–59.
Inoue D, Bradley R, Abdel-Wahab O. Spliceosomal Gene Mutations in Myelodysplasia: molecular Links to Clonal Abnormalities of Hematopoiesis. Genes Dev. 2016;30:989–1001.
Wu S, Romfo CM, Nilsen TW, Green MR. Functional Recognition of the 3’ Splice Site AG by the Splicing Factor U2AF35. Nature. 1999;402:832–35.
Makishima H, Visconte V, Sakaguchi H, Jankowska AM, Abu Kar S, Jerez A, et al. Mutations in the spliceosome machinery, a novel and ubiquitous pathway in leukemogenesis. Blood. 2012;119:3203–10.
Przychodzen B, Jerez A, Guinta K, Sekeres MA, Padgett R, Maciejewski JP, et al. Patterns of Missplicing Due to Somatic U2AF1 Mutations in Myeloid Neoplasms. Blood. 2013;122:999–1006.
Ilagan JO, Ramakrishnan A, Hayes B, Murphy ME, Zebari AS, Bradley P, et al. U2AF1 Mutations Alter Splice Site Recognition in Hematological Malignancies. Genome Res. 2015;25:14–26.
Brooks AN, Choi P, de Waal L, Sharifnia T, Imielinski M, Saksena G, et al. A Pan-Cancer Analysis of Transcriptome Changes Associated With Somatic Mutations in U2AF1 Reveals Commonly Altered Splicing Events. PLoS ONE. 2014;9:e87361.
Okeyo-Owuor T, White BS, Chatrikhi R, Mohan DR, Kim S, Griffith M, et al. U2AF1 mutations alter sequence specificity of pre-mRNA binding and splicing. Leukemia. 2015;29:909–17.
Yip BH, Steeples V, Repapi E, Armstrong RN, Llorian M, Roy S, et al. The U2AF1S34F Mutation Induces Lineage-Specific Splicing Alterations in Myelodysplastic Syndromes. J Clin Investig. 2017;127:2206–21.
Shirai CL, Ley JN, White BS, Kim S, Tibbitts J, Shao J, et al. Mutant U2AF1 Expression Alters Hematopoiesis and Pre-mRNA Splicing In Vivo. Cancer Cell. 2015;27:631–43.
Fei DL, Zhen T, Durham B, Ferrarone J, Zhang T, Garrett L, et al. Impaired hematopoiesis and leukemia development in mice with a conditional knock-in allele of a mutant splicing factor gene U2af1. Proc Natl Acad Sci USA. 2018;115:E10437–46.
Kühn R, Schwenk F, Aguet M, Rajewsky K. Inducible gene targeting in mice. Science. 1995;269:1427–29.
Lee Y, Wang Q, Shuryak I, Brenner DJ, Turner HC. Development of a High-Throughput γ-H2AX Assay Based on Imaging Flow Cytometry. Radiat Oncol. 2019;14:150.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 2005;102:15545–50.
Shen S, Park JW, Lu ZX, Lin L, Henry MD, Wu YN, et al. rMATS: robust and flexible detection of differential alternative splicing from replicate RNA-Seq data. Proc Natl Acad Sci USA 2014;111:E5593–601.
Chen L, Chen J, Huang YJ, Gu Y, Qiu J, Qian H, et al. The Augmented R-Loop Is a Unifying Mechanism for Myelodysplastic Syndromes Induced by High-Risk Splicing Factor Mutations. Mol Cell. 2018;69:412–25.
Nguyen HD, Leon W, Li W, Reddy PNG, Sullivan JD, Walter MJ, et al. Spliceosome Mutations Induce R Loop-Associated Sensitivity to ATR Inhibition in Myelodysplastic Syndromes. Cancer Res. 2018;78:5363–74.
Zhu J, Zhang Y, Joe GJ, Pompetti R, Emerson SG. NF-Ya Activates Multiple Hematopoietic Stem Cell (HSC) Regulatory Genes and Promotes HSC Self-Renewal. Proc Natl Acad Sci USA 2005;102:11728–33.
Bungartz G, Land H, Scadden DT, Emerson SG. NF-Y Is Necessary for Hematopoietic Stem Cell Proliferation and Survival. Blood. 2012;119:1380–89.
Ficara F, Murphy MJ, Lin M, Cleary ML. Pbx1 regulates self-renewal of long-term hematopoietic stem cells by maintaining their quiescence. Cell Stem Cell. 2008;2:484–96.
Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–12.
Mackarehtschian K, Hardin JD, Moore KA, Boast S, Goff SP, Lemischka IR. Targeted Disruption of the flk2/flt3 Gene Leads to Deficiencies in Primitive Hematopoietic Progenitors. Immunity. 1995;3:147–61.
Kuo YH, Zaidi SK, Gornostaeva S, Komori T, Stein GS, Castilla LH. Runx2 Induces Acute Myeloid Leukemia in Cooperation With Cbfbeta-SMMHC in Mice. Blood. 2009;113:3323–32.
Unnisa Z, Clark JP, Roychoudhury J, Thomas E, Tessarollo L, Copeland NG, et al. Meis1 Preserves Hematopoietic Stem Cells in Mice by Limiting Oxidative Stress. Blood. 2012;120:4973–81.
Ariki R, Morikawa S, Mabuchi Y, Suzuki S, Nakatake M, Yoshioka K, et al. Homeodomain Transcription Factor Meis1 Is a Critical Regulator of Adult Bone Marrow Hematopoiesis. PLoS ONE. 2014;9:e87646.
Folco EG, Coli KE, Reed R. The Anti-Tumor Drug E7107 Reveals an Essential Role for SF3b in Remodeling U2 snRNP to Expose the Branch Point-Binding Region. Genes Dev. 2011;25:440–44.
Eskens FA, Ramos F, Burger H, O’Brien JP, Piera A, de Jonge MJ, et al. Phase I Pharmacokinetic and Pharmacodynamic Study of the First-In-Class Spliceosome Inhibitor E7107 in Patients With Advanced Solid Tumors. Clin Cancer Res. 2013;19:6296–04.
Lee SC-W, Abdel-Wahab O. Therapeutic Targeting of Splicing in Cancer. Nat Med. 2016;22:976–86.
Shirai CL, White BS, Tripathi M, Tapia R, Ley JN, Ndonwi M, et al. Mutant U2AF1-expressing Cells Are Sensitive to Pharmacological Modulation of the Spliceosome. Nat Commun. 2017;8:14060.
Seiler M, Yoshimi A, Darman R, Chan B, Keaney G, Thomas M, et al. H3B-8800, an Orally Available Small-Molecule Splicing Modulator, Induces Lethality in Spliceosome-Mutant Cancers. Nat Med. 2018;24:497–504.
Acknowledgements
We thank Dr Roberto Mantovani (University of Milan) and Dr Licia Selleri (University of California San Francisco) for NF-Ya and Pbx1 expression constructs, and Ms Julia Dreksler for assistance with generating U2AF1 knockdown cell lines. We also thank the flow cytometry and microscopy core facilities at the University of Virginia for assistance with FACS sorting and confocal microscopy. Flow cytometry and microscopy cores are supported by the UVA Cancer Center through NCI P30CA044578 grant. This work was supported in part by US National Institute of Health (NIH) grants R01 HL095685 (GM), R35 GM133712 (CZ) and a start-up fund from the Department of Biochemistry and Molecular Genetics of the University of Virginia (GM).
Author information
Authors and Affiliations
Contributions
AD performed research, analyzed data and wrote the paper; YY performed research; BTL performed data analysis; YZ performed data analysis; OAW provided critical advice on genomic data analysis and revised the paper; CZ performed data analysis; GM designed the research, analyzed data, and prepared the paper with the help from co-authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Dutta, A., Yang, Y., Le, B.T. et al. U2af1 is required for survival and function of hematopoietic stem/progenitor cells. Leukemia 35, 2382–2398 (2021). https://doi.org/10.1038/s41375-020-01116-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-020-01116-x
This article is cited by
-
Inhibitor of DNA Binding Protein 3 (ID3) and Nuclear Respiratory Factor 1 (NRF1) Mediated Transcriptional Gene Signatures are Associated with the Severity of Cerebral Amyloid Angiopathy
Molecular Neurobiology (2024)
-
Single-cell RNA sequencing distinctly characterizes the wide heterogeneity in pediatric mixed phenotype acute leukemia
Genome Medicine (2023)
-
NF-YAl drives EMT in Claudinlow tumours
Cell Death & Disease (2023)