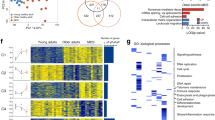
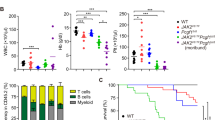
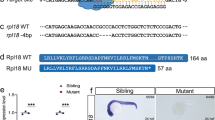
Abstract
EZH1 and EZH2 are enzymatic components of polycomb repressive complex (PRC) 2, which catalyzes histone H3K27 tri-methylation (H3K27me3) to repress the transcription of PRC2 target genes. We previously reported that the hematopoietic cell-specific Ezh2 deletion (Ezh2Δ/Δ) induced a myelodysplastic syndrome (MDS)-like disease in mice. We herein demonstrated that severe PRC2 insufficiency induced by the deletion of one allele Ezh1 in Ezh2-deficient mice (Ezh1+/−Ezh2Δ/Δ) caused advanced dyserythropoiesis accompanied by a differentiation block and enhanced apoptosis in erythroblasts. p53, which is activated by impaired ribosome biogenesis in del(5q) MDS, was specifically activated in erythroblasts, but not in hematopoietic stem or progenitor cells in Ezh1+/−Ezh2Δ/Δ mice. Cdkn2a, a major PRC2 target encoding p19Arf, which activates p53 by inhibiting MDM2 E3 ubiquitin ligase, was de-repressed in Ezh1+/−Ezh2Δ/Δ erythroblasts. The deletion of Cdkn2a as well as p53 rescued dyserythropoiesis in Ezh1+/−Ezh2Δ/Δ mice, indicating that PRC2 insufficiency caused p53-dependent dyserythropoiesis via the de-repression of Cdkn2a. Since PRC2 insufficiency is often involved in the pathogenesis of MDS, the present results suggest that p53-dependent dyserythropoiesis manifests in MDS in the setting of PRC2 insufficiency.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Margueron R, Reinberg D. The Polycomb complex PRC2 and its mark in life. Nature. 2011;469:343–9.
Blackledge NP, Rose NR, Klose RJ. Targeting polycomb systems to regulate gene expression: modifications to a complex story. Nat Rev Mol Cell Biol. 2015;16:643–9.
Iwama A. Polycomb repressive complexes in hematological malignancies. Blood. 2017;130:23–9.
Sashida G, Iwama A. Multifaceted role of the polycomb-group gene EZH2 in hematological malignancies. Int J Hematol. 2017;105:23–30.
Mochizuki-Kashio M, Aoyama K, Sashida G, Oshima M, Tomioka T, Muto T, et al. Ezh2 loss in hematopoietic stem cells predisposes mice to develop heterogeneous malignancies in an Ezh1-dependent manner. Blood. 2015;126:1172–83.
Aoyama K, Oshima M, Koide S, Suzuki E, Mochizuki-Kashio M, Kato Y. et al. Ezh1 targets bivalent genes maint self-renewing stem cells Ezh2-insufficient myelodysplastic syndrome. iScience. 2018;9:161–74.
Heaney ML, Golde DW. Myelodysplasia. N Engl J Med. 1999;340:1649–60.
Ebert BL, Pretz J, Bosco J, Chang CY, Tamayo P, Galili N, et al. Identification of RPS14 as a 5q- syndrome gene by RNA interference screen. Nature. 2008;451:335–9.
Barlow JL, Drynan LF, Hewett DR, Holmes LR, Lorenzo-Abalde S, Lane AL, et al. A p53-dependent mechanism underlies macrocytic anemia in a mouse model of human 5q- syndrome. Nat Med. 2010;16:59–66.
Pellagatti A, Marafioti T, Paterson JC, Barlow JL, Drynan LF, Giagounidis A, et al. Induction of p53 and up-regulation of the p53 pathway in the human 5q- syndrome. Blood. 2010;115:2721–3.
Dutt S, Narla A, Lin K, Mullally A, Abayasekara N, Megerdichian C, et al. Haploinsufficiency for ribosomal protein genes causes selective activation of p53 in human erythroid progenitor cells. Blood. 2011;117:2567–76.
McGowan KA, Pang WW, Bhardwaj R, Perez MG, Pluvinage JV, Glader BE, et al. Reduced ribosomal protein gene dosage and p53 activation in low-risk myelodysplastic syndrome. Blood. 2011;118:3622–33.
Hirabayashi Y, Suzki N, Tsuboi M, Endo TA, Toyoda T, Shinga J, et al. Polycomb limits the neurogenic competence of neural precursor cells to promote astrogenic fate transition. Neuron. 2009;63:600–13.
Jonkers J, Meuwissen R, van der Gulden H, Peterse H, van der Valk M, Berns A. Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat Genet. 2001;29:418–25.
Ezhkova E, Lien WH, Stokes N, Pasolli HA, Silva JM, Fuchs E. EZH1 and EZH2 cogovern histone H3K27 trimethylation and are essential for hair follicle homeostasis and wound repair. Genes Dev. 2011;25:485–98.
Bae WK, Kang K, Yu JH, Yoo KH, Factor VM, Kaji K, et al. The methyltransferases enhancer of zeste homolog (EZH) 1 and EZH2 control hepatocyte homeostasis and regeneration. FASEB J. 2015;29:1653–62.
Oguro H, Iwama A, Morita Y, Kamijo T, van Lohuizen M, Nakauchi H. Differential impact of Ink4a and Arf on hematopoietic stem cells and their bone marrow microenvironment in Bmi1-deficient mice. J Exp Med. 2006;203:2247–53.
Tanaka T, Nakajima-Takagi Y, Aoyama K, Tara S, Oshima M, Saraya A, et al. Internal deletion of BCOR reveals a tumor suppressor function for BCOR in T lymphocyte malignancies. J Exp Med. 2017;214:2901–13.
Egan B, Yuan CC, Craske ML, Labhart P, Guler GD, Arnott D, et al. An alternative approach to Chip-Seq normalization enables detection of genome-wide changes in histone H3 lysine 27 trimethylation upon EZH2 inhibition. PloS One 2016;11:e0166438.
Schneider RK, Schenone M, Ferreira MV, Kramann R, Joyce CE, Hartigan C, et al. Rps14 haploinsufficiency causes a block in erythroid differentiation mediated by S100A8 and S100A9. Nat Med. 2016;22:288–97.
Zhang Y, Xiong Y, Yarbrough WG. ARF promotes MDM2 degradation and stabilizes p53: ARF-INK4a locus deletion impairs both the Rb and p53 tumor suppression pathways. Cell. 1998;92:725–34.
Dai MS, Challagundla KB, Sun XX, Palam LR, Zeng SX, Wek RC, et al. Physical and functional interaction between ribosomal protein L11 and the tumor suppressor ARF. J Biol Chem. 2012;287:17120–9.
Donati G, Peddigari S, Mercer CA, Thomas G. 5S ribosomal RNA is an essential component of a nascent ribosomal precursor complex that regulates the Hdm2-p53 checkpoint. Cell Rep. 2013;4:87–98.
Sloan KE, Bohnsack MT, Watkins NJ. The 5S RNP couples p53 homeostasis to ribosome biogenesis and nucleolar stress. Cell Rep. 2013;5:237–47.
Holoch D, Margueron R. Mechanisms regulating PRC2 recruitment and enzymatic activity. Trends Biochem Sci. 2017;42:531–42.
Deevy O, Bracken AP. PRC2 functions in development and congenital disorders. Development. 2019;146:dev181354.
Laugesen A, Højfeldt JW, Helin K. Molecular mechanisms directing PRC2 recruitment and H3K27 methylation. Mol Cell. 2019;74:8–18.
Yu JR, Lee CH, Oksuz O, Stafford JM, Reinberg D. PRC2 is high maintenance. Genes Dev. 2019;33:903–35.
Tara S, Isshiki Y, Nakajima-Takagi Y, Oshima M, Aoyama K, Tanaka T, et al. Bcor insufficiency promotes initiation and progression of myelodysplastic syndrome. Blood. 2018;132:2470–83.
Acknowledgements
Ezh1 constitutive knockout mice were generated at the Research Institute of Molecular Pathology (Vienna, Austria) in 2000 by Donal O’Carroll (Laboratory Thomas Jenuwein) with the help of Maria Sibilia (Laboratory Erwin Wagner). Ezh2fl/fl and p53fl/fl mice were kindly provided by Haruhiko Koseki (RIKEN, Japan) and by Anton Berns (Netherlands Cancer Institute), respectively. The authors thank Yuko Yamagata for her technical help and Chikako Furuta and Mohamed Rizk for critical review of our paper. The super-computing resource was provided by the Human Genome Center, The Institute of Medical Science, The University of Tokyo. This work was supported in part by Grants-in-Aid for Scientific Research (#19H05653) and Scientific Research on Innovative Areas “Stem Cell Aging and Disease” (#26115002) and “Replication of Non-Genomic Codes” (#19H05746) from MEXT, Japan.
Author information
Authors and Affiliations
Contributions
KA and DS performed the experiments, analyzed results, made the figures, and actively wrote the paper; ES, YN-T, MO, SK, OR, SS, ST, and GS assisted with experiments; and AI conceived of and directed the project, secured funding, and actively wrote the paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no competing financial interests to declare.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Aoyama, K., Shinoda, D., Suzuki, E. et al. PRC2 insufficiency causes p53-dependent dyserythropoiesis in myelodysplastic syndrome. Leukemia 35, 1156–1165 (2021). https://doi.org/10.1038/s41375-020-01023-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-020-01023-1