Abstract
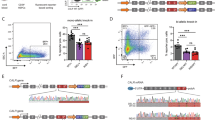
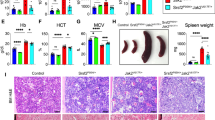
Frameshifting mutations (−1/+2) of the calreticulin (CALR) gene are responsible for the development of essential thrombocythemia (ET) and primary myelofibrosis (PMF). The mutant CALR proteins activate the thrombopoietin receptor (TpoR) inducing cytokine-independent megakaryocyte progenitor proliferation. Here, we generated via CRISPR/Cas9 technology two knock-in mouse models that are heterozygous for a type-I murine Calr mutation. These mice exhibit an ET phenotype with elevated circulating platelets compared with wild-type controls, consistent with our previous results showing that murine CALR mutants activate TpoR. We also show that the mutant CALR proteins can be detected in plasma. The phenotype of Calr del52 is transplantable, and the Calr mutated hematopoietic cells have a slow-rising advantage over wild-type hematopoiesis. Importantly, a homozygous state of a type-1 Calr mutation is lethal at a late embryonic development stage, showing narrowed ventricular myocardium walls, similar to the murine Calr knockout phenotype, pointing to the C terminus of CALR as crucial for heart development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391.
Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–90.
James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–8.
Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–97.
Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–61.
Klampfl T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, Milosevic JD, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369:2379–90.
Nangalia J, Massie CE, Baxter EJ, Nice FL, Gundem G, Wedge DC, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med. 2013;369:2391–405.
Chachoua I, Pecquet C, El-Khoury M, Nivarthi H, Albu RI, Marty C, et al. Thrombopoietin receptor activation by myeloproliferative neoplasm associated calreticulin mutants. Blood. 2016;127:1325–35.
Araki M, Yang Y, Masubuchi N, Hironaka Y, Takei H, Morishita S, et al. Activation of the thrombopoietin receptor by mutant calreticulin in CALR-mutant myeloproliferative neoplasms. Blood. 2016;127:1307–16.
Elf S, Abdelfattah NS, Chen E, Perales-Paton J, Rosen EA, Ko A, et al. Mutant calreticulin requires both its mutant C-terminus and the thrombopoietin receptor for oncogenic transformation. Cancer Discov. 2016;6:368–81.
Elf S, Abdelfattah NS, Baral AJ, Beeson D, Rivera JF, Ko A, et al. Defining the requirements for the pathogenic interaction between mutant calreticulin and MPL in MPN. Blood. 2018;131:782–6.
Marty C, Pecquet C, Nivarthi H, El-Khoury M, Chachoua I, Tulliez M, et al. Calreticulin mutants in mice induce an MPL-dependent thrombocytosis with frequent progression to myelofibrosis. Blood. 2016;127:1317–24.
Nivarthi H, Chen D, Cleary C, Kubesova B, Jager R, Bogner E, et al. Thrombopoietin receptor is required for the oncogenic function of CALR mutants. Leukemia. 2016;30:1759–63.
Han L, Schubert C, Kohler J, Schemionek M, Isfort S, Brummendorf TH, et al. Calreticulin-mutant proteins induce megakaryocytic signaling to transform hematopoietic cells and undergo accelerated degradation and Golgi-mediated secretion. J Hematol Oncol. 2016;9:45.
Pecquet C, Chachoua I, Roy A, Balligand T, Vertenoeil G, Leroy E, et al. Calreticulin mutants as oncogenic rogue chaperones for TpoR and traffic-defective pathogenic TpoR mutants. Blood. 2019;133:2669–81.
Balligand T, Achouri Y, Pecquet C, Chachoua I, Nivarthi H, Marty C, et al. Pathologic activation of thrombopoietin receptor and JAK2-STAT5 pathway by frameshift mutants of mouse calreticulin. Leukemia. 2016;30:1775–8.
Shide K, Kameda T, Yamaji T, Sekine M, Inada N, Kamiunten A, et al. Calreticulin mutant mice develop essential thrombocythemia that is ameliorated by the JAK inhibitor ruxolitinib. Leukemia. 2016;31:1136–44.
Li J, Prins D, Park HJ, Grinfeld J, Gonzalez-Arias C, Loughran S, et al. Mutant calreticulin knock-in mice develop thrombocytosis and myelofibrosis without a stem cell self-renewal advantage. Blood. 2018;131:649–61.
Shide K, Kameda T, Kamiunten A, Oji A, Ozono Y, Sekine M, et al. Mice with Calr mutations homologous to human CALR mutations only exhibit mild thrombocytosis. Blood Cancer J. 2019;9:42.
Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–23.
Mashiko D, Fujihara Y, Satouh Y, Miyata H, Isotani A, Ikawa M. Generation of mutant mice by pronuclear injection of circular plasmid expressing Cas9 and single guided RNA. Sci Rep. 2013;3:3355.
Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A Programmable Dual-RNA–Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science. 2012;337:816–21.
Balligand T, Achouri Y, Chachoua I, Pecquet C, Defour J-P, Constantinescu SN. Crispr/Cas9 engineered 61bp deletion in the Calr gene of mice leads to development of thrombocytosis. Blood. 2016;128:4274-.
Kollmann K, Nangalia J, Warsch W, Quentmeier H, Bench A, Boyd E, et al. MARIMO cells harbor a CALR mutation but are not dependent on JAK2/STAT5 signaling. Leukemia. 2015;29:494–7.
Yoshida H, Kondo M, Ichihashi T, Hashimoto N, Inazawa J, Ohno R, et al. A novel myeloid cell line, Marimo, derived from therapy-related acute myeloid leukemia during treatment of essential thrombocythemia: consistent chromosomal abnormalities and temporary C-MYC gene amplification. Cancer Genet Cytogenet. 1998;100:21–4.
Han L, Czech J, Maurer A, Brummendorf TH, Chatain N, Koschmieder S. Mutant NRAS Q61K is responsible for MAPK pathway activation in the MARIMO cell line and renders these cells independent of the CALR-MPL-JAK2-STAT5 pathway. Leukemia. 2018;32:2087–90.
Staerk J, Defour JP, Pecquet C, Leroy E, Antoine-Poirel H, Brett I, et al. Orientation-specific signalling by thrombopoietin receptor dimers. Embo J. 2011;30:4398–413.
Defour JP, Itaya M, Gryshkova V, Brett IC, Pecquet C, Sato T, et al. Tryptophan at the transmembrane-cytosolic junction modulates thrombopoietin receptor dimerization and activation. Proc Natl Acad Sci USA. 2013;110:2540–5.
Leroy E, Defour JP, Sato T, Dass S, Gryshkova V, Shwe MM, et al. His499 regulates dimerization and prevents oncogenic activation by asparagine mutations of the human thrombopoietin receptor. J Biol Chem. 2016;291:2974–87.
Luoh SM, Stefanich E, Solar G, Steinmetz H, Lipari T, Pestina TI, et al. Role of the distal half of the c-Mpl intracellular domain in control of platelet production by thrombopoietin in vivo. Mol Cell Biol. 2000;20:507–15.
Garbati MR, Welgan CA, Landefeld SH, Newell LF, Agarwal A, Dunlap JB, et al. Mutant calreticulin-expressing cells induce monocyte hyperreactivity through a paracrine mechanism. Am J Hematol. 2016;91:211–9.
Arshad N, Cresswell P. Tumor-associated calreticulin variants functionally compromise the peptide loading complex and impair its recruitment of MHC-I. J Biol Chem. 2018;293:9555–69.
Pecquet C, Balligand T, Chachoua I, Roy A, Vertenoeil G, Colau D, et al. Secreted mutant calreticulins as rogue cytokines trigger thrombopoietin receptor activation specifically in CALR mutated cells: perspectives for mpn therapy. Blood. 2018;132:4.
Pecquet C, Chachoua I, Roy A, Balligand T, Vertenoeil G, Defour J-P, et al. MPN Calr mutants promote cell-surface localization of tpor which is obligatory for oncogenesis: novel therapeutic avenues and rescue of congenital thrombocytopenia TpoR mutants. EHA Learning Center. 2018:215926. https://learningcenter.ehaweb.org/eha/2018/stockholm/215926/christian.pecquet.mpn.calr.mutants.promote.cell-surface.localization.of.tpor.html?f=topic=1602*media=3*search=pecquet*listing=6*browseby=8.
Mesaeli N, Nakamura K, Zvaritch E, Dickie P, Dziak E, Krause KH, et al. Calreticulin is essential for cardiac development. J Cell Biol. 1999;144:857–68.
Rauch F, Prud’homme J, Arabian A, Dedhar S, St-Arnaud R. Heart, brain, and body wall defects in mice lacking calreticulin. Exp Cell Res. 2000;256:105–11.
Rumi E, Pietra D, Ferretti V, Klampfl T, Harutyunyan AS, Milosevic JD, et al. JAK2 or CALR mutation status defines subtypes of essential thrombocythemia with substantially different clinical course and outcomes. Blood. 2014;123:1544–51.
Tefferi A, Lasho TL, Finke CM, Knudson RA, Ketterling R, Hanson CH, et al. CALR vs JAK2 vs MPL-mutated or triple-negative myelofibrosis: clinical, cytogenetic and molecular comparisons. Leukemia. 2014;28:1472–7.
Michalak M, Corbett EF, Mesaeli N, Nakamura K, Opas M. Calreticulin: one protein, one gene, many functions. Biochem J. 1999;344:281–92.
Perry JS, Hsieh CS. Development of T-cell tolerance utilizes both cell-autonomous and cooperative presentation of self-antigen. Immunol Rev. 2016;271:141–55.
Hartley SB, Cooke MP, Fulcher DA, Harris AW, Cory S, Basten A, et al. Elimination of self-reactive B lymphocytes proceeds in two stages: Arrested development and cell death. Cell. 1993;72:325–35.
Tubb VM, Schrikkema DS, Croft NP, Purcell AW, Linnemann C, Freriks MR, et al. Isolation of T cell receptors targeting recurrent neoantigens in hematological malignancies. J Immunother Cancer. 2018;6:70.
Guo L, Nakamura K, Lynch J, Opas M, Olson EN, Agellon LB, et al. Cardiac-specific expression of calcineurin reverses embryonic lethality in calreticulin-deficient mouse. J Biol Chem. 2002;277:50776–9.
Acknowledgements
We thank Dr Patrick Jacquemin for advice on CRISPR/Cas9 and transgenesis, Dr Pedro Gomez for support with mouse facility, Dr Nicolas Dauguet for expert flow cytometry support, Dr Jing Jing Zhu for her help with the T-cell intracellular staining assay, Lidvine Genet and Céline Mouton for expert technical and administrative support. TB was supported by a Télévie PhD Fellowship. SNC is Honorary Research Director at FRS-FNRS Belgium. Funding to SNC is acknowledged from Ludwig Institute for Cancer Research, Fondation contre le cancer, Fondation « Les avions de Sébastien », Fondation Salus Sanguinis and projects Action de Recherche Concertée (ARC) 16/21-073 and WelBio (Walloon Excellence In Life Sciences and Biotechnology F 44/8/5- MCF/UIG-10955), Brussels, Belgium.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
RK and SNC are cofounders of MyeloPro Research and Diagnostics GmbH, Vienna, Austria. The remaining authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Balligand, T., Achouri, Y., Pecquet, C. et al. Knock-in of murine Calr del52 induces essential thrombocythemia with slow-rising dominance in mice and reveals key role of Calr exon 9 in cardiac development. Leukemia 34, 510–521 (2020). https://doi.org/10.1038/s41375-019-0538-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-019-0538-1
This article is cited by
-
Differential in vivo roles of Mpl cytoplasmic tyrosine residues in murine hematopoiesis and myeloproliferative disease
Leukemia (2024)
-
CALR-mutated cells are vulnerable to combined inhibition of the proteasome and the endoplasmic reticulum stress response
Leukemia (2023)
-
Human gene-engineered calreticulin mutant stem cells recapitulate MPN hallmarks and identify targetable vulnerabilities
Leukemia (2023)
-
Calreticulin: a quintessential multifaceted protein with therapeutic potential
Journal of Proteins and Proteomics (2023)
-
Calreticulin del52 and ins5 knock-in mice recapitulate different myeloproliferative phenotypes observed in patients with MPN
Nature Communications (2020)