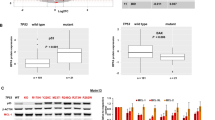
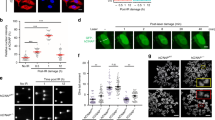
Abstract
The E3 ligase human double minute 2 (HDM2) regulates the activity of the tumor suppressor protein p53. A p53-independent HDM2 expression has been reported on the membrane of cancer cells but not on that of normal cells. Herein, we first showed that membrane HDM2 (mHDM2) is exclusively expressed on human and mouse AML blasts, including leukemia stem cell (LSC)-enriched subpopulations, but not on normal hematopoietic stem cells (HSCs). Higher mHDM2 levels in AML blasts were associated with leukemia-initiating capacity, quiescence, and chemoresistance. We also showed that a synthetic peptide PNC-27 binds to mHDM2 and enhances the interaction of mHDM2 and E-cadherin on the cell membrane; in turn, E-cadherin ubiquitination and degradation lead to membrane damage and cell death of AML blasts by necrobiosis. PNC-27 treatment in vivo resulted in a significant killing of both AML “bulk” blasts and LSCs, as demonstrated respectively in primary and secondary transplant experiments, using both human and murine AML models. Notably, PNC-27 spares normal HSC activity, as demonstrated in primary and secondary BM transplant experiments of wild-type mice. We concluded that mHDM2 represents a novel and unique therapeutic target, and targeting mHDM2 using PNC-27 selectively kills AML cells, including LSCs, with minimal off-target hematopoietic toxicity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ishikawa F, Yoshida S, Saito Y, Hijikata A, Kitamura H, Tanaka S, et al. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol. 2007;25:1315–21.
Gentles AJ, Plevritis SK, Page P, Alizadeh AA. Association of a leukemic stem cell gene expression signature with clinical outcome in acute myeloid leukemia. JAMA. 2012;304:2706–15.
Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell Stem Cell. 2014;14:275–91.
Alarcon-Vargas D, Ronai Z. p53-Mdm2-the affair that never ends. Carcinogenesis. 2002;23:541–7.
Watanabe T, Ichikawa A, Saito H, Hotta T. Overexpression of the MDM2 oncogene in leukemia and lymphoma. Leuk Lymphoma. 1996;21:391–7.
Capoulade C, Bressac-de Paillerets B, Lefrere I, Ronsin M, Feunteun J, Tursz T. et al. Overexpression of MDM2, due to enhanced translation, results in inactivation of wild-type p53 in Burkitt’s lymphoma cells. Oncogene. 1998;16:1603–10.
Bueso-Ramos CE, Manshouri T, Haidar MA, Yang Y, McCown P, Ordonez N, et al. Abnormal expression of MDM-2 in breast carcinomas. Breast Cancer Res Treat. 1996;37:179–88.
Polsky D, Bastian BC, Hazan C, Melzer K, Pack J, Houghton A, et al. HDM2 protein overexpression, but not gene amplification, is related to tumorigenesis of cutaneous melanoma. Cancer Res. 2001;61:7642–6.
Momand J, Jung D, Wilczynski S, Niland J. The MDM2 gene amplification database. Nucleic Acids Res. 1998;26:3453–9.
Fakharzadeh SS, Trusko SP, George DL. Tumorigenic potential associated with enhanced expression of a gene that is amplified in a mouse tumor cell line. EMBO J. 1991;10:1565–9.
Jones SN, Hancock AR, Vogel H, Donehower LA, Bradley A. Overexpression of Mdm2 in mice reveals a p53-independent role for Mdm2 in tumorigenesis. Proc Natl Acad Sci USA. 1998;95:15608–12.
Sarafraz-Yazdi E, Bowne WB, Adler V, Sookraj KA, Wu V, Shteyler V, et al. Anticancer peptide PNC-27 adopts an HDM-2-binding conformation and kills cancer cells by binding to HDM-2 in their membranes. Proc Natl Acad Sci USA. 2010;107:1918–23.
Kanovsky M, Raffo A, Drew L, Rosal R, Do T, Friedman FK, et al. Peptides from the amino terminal mdm-2-binding domain ofp53, designed from conformational analysis, are selectively cytotoxic to transformed cells. Proc Natl Acad Sci USA. 2001;98:12438–43.
Bowne WB, Sookraj KA, Vishnevetsky M, Adler V, Sarafraz-Yazdi E, Lou S. The penetratin sequence in the anticancer PNC-28 peptide causes tumor cell necrosis rather than apoptosis of human pancreatic cancer cells. Ann Surg Oncol. 2008;15:3588–3600.
Rosal R, Pincus MR, Brandt-Rauf PW, Fine RL, Michl J, Wang H. NMR solution structure of a peptide from the mdm-2 binding domain of the p53 protein that is selectively cytotoxic to cancer cells. Biochemistry. 2004;43:1854–61.
Michl J, Scharf B, Schmidt A, Huynh C, Hannan R, von Gizycki H, et al. PNC-28, a p53-derived peptide that is cytotoxic to cancer cells, blocks pancreatic cancer cell growth in vivo. Int J Cancer. 2006;119:1577–85.
Davitt K, Babcock BD, Fenelus M, Poon CK, Sarkar A, Trivigno V, et al. The anti-cancer peptide, PNC-27, induces tumor cell necrosis of a poorly differentiated non-solid tissue human leukemia cell line that depends on expression of HDM-2 in the plasma membrane of these cells. Ann Clin Lab Sci. 2014;44:241–8.
Sookraj KA, Bowne WB, Adler V, Sarafraz-Yazdi E, Michl J, Pincus MR. The anti-cancer peptide, PNC-27, induces tumor cell lysis as the intact peptide. Cancer Chemother Pharm. 2010;66:325–31.
Pincus MR. The physiological structure and function of proteins. In: Sperelakis N editors. Principles of Cell Physiology. 3rd ed. New York, NY, USA: Academic press; 2001. pp 19–42.
Dathe M, Wieprecht T. Structural features of helical anti-microbial peptides: their potential to modulate activity on model membranes and biological cells. Biochem Biophys Acta. 1999;1462:71–87.
Palmer M, Valeva A, Kehoe M, Bhakdi S. Kinetics of streptolysin O self-assembly. Eur J Biochem. 1995;231:388–95.
Pincus MR, Fenelus M, Sarafraz-Yazdi E, Adler V, Bowne W, Michl J. Anti-cancer peptides from ras-p21 and p53 proteins. Curr Pharm Des. 2011;17:2677–98.
Li L, Osdal T, Ho Y, Chun S, McDonald T, Agarwal P, et al. SIRT1 activation by a c-MYC oncogenic network promotes the maintenance and drug resistance of human FLT3-ITD acute myeloid leukemia stem cells. Cell Stem Cell. 2014;15:431–46.
Bhatia R, McGlave PB, Dewald GW, Blazar BR, Verfaillie CM. Abnormal function of the bone marrow microenvironment in chronic myelogenous leukemia: role of malignant stromal macrophages. Blood. 1995;85:3636–45.
Zhang B, Nguyen LXT, Li L, Zhao D, Kumar B, Wu H, et al. Bone marrow niche trafficking of miR-126 controls the self-renewal of leukemia stem cells in chronic myelogenous leukemia. Nat Med. 2018;24:450–62.
Zorko NA, Bernot KM, Whitman SP, Siebenaler RF, Ahmed EH, Marcucci GG, et al. Mll partial tandem duplication and Flt3 internal tandem duplication in a double knock-in mouse recapitulates features of counterpart human acute myeloid leukemias. Blood. 2012;120:1130–6.
Yang JY, Zong CS, Xia W, Wei Y, Ali-Seyed M, Li Z, et al. MDM2 promotes cell motility and invasiveness by regulating E-cadherin degradation. Mol Cell Biol. 2006;26:7269–82.
Taneyhill LA, Schiffmacher AT. Should I stay or should I go? Cadherin function and regulation in the neural crest. Genesis. 2017;55:1–39.
Ng SW, Mitchell A, Kennedy JA, Chen WC, McLeod J, Ibrahimova N, et al. A 17-gene stemness score for rapid determination of risk in acute leukaemia. Nature. 2016;540:433–7.
Darban SA, Badiee A, Jaafari MR. PNC27 anticancer peptide as targeting ligand significantly improved efficacy of Doxil in HDM2-expressiong cells. Nanomedicine. 2017;12:1475–90.
Al-toub M, Vishnubalaji R, Hamam R, Kassem M, Aldahmash A, Alajez NM. CDH1 and IL1-beta expression dictates FAK and MAPKK-dependent cross-talk between cancer cells and human mesenchymal stem cells. Stem Cell Res Ther. 2015;6:135.
Nishioka C, Ikezoe T, Pan B, Xu K, Yokoyama A. MicroRNA-9 plays a role in interleukin-10-mediated expression of E-cadherin in acute myelogenous leukemia cells. Cancer Sci. 2017;108:685–95.
Ewerth D, Schmidts A, Hein M, et al. Suppression of APC/CCdh1 has subtype specific biological effects in acute myeloid leukemia. Oncotarget. 2016;7:48220–30.
Acknowledgements
This work was supported in part by National Cancer Institute grants: CA102031 (GM), CA201184 (GM), CA180861 (GM), and CA158350 (GM); the Gehr Family Foundation; Youth Natural Science Foundation of Zhejiang Province, China (LQ18H080001); National Natural Science Foundation of China (no. 81370643); Oncolyze, Inc. (drug supply). Research reported in this publication included work performed in the Animal Resources Center, Analytical Cytometry, Hematopoietic Tissue Bank Core, and Electron Microscopy and Light Microscopy Cores at City of Hope Comprehensive Cancer Center supported by the National Cancer Institute of the National Institutes of Health under award number P30CA33572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We are grateful to COH Comprehensive Cancer Center, the patients, and their physicians for providing primary patient material for this study.
Author information
Authors and Affiliations
Contributions
HFW designed and conducted experiments, analyzed the data, and wrote the paper; DDZ, LXN, HW, LL, DD, ET, YHZ, DHH, and WXM conducted experiments; ASS, MAM, IA, AL, LYG, and TM provided samples and reviewed the patients’ data; FP, NC, and Y-HK designed experiments and reviewed the paper; BZ designed and conducted experiments, analyzed the data, and wrote paper; JJ and GM designed experiments, analyzed the data, wrote the paper, and provided administrative support.
Corresponding authors
Ethics declarations
Conflict of interest
Oncolyze, Inc. provided PNC-27, PNC-26, and PNC-29 for this study. The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Wang, H., Zhao, D., Nguyen, L.X. et al. Targeting cell membrane HDM2: A novel therapeutic approach for acute myeloid leukemia. Leukemia 34, 75–86 (2020). https://doi.org/10.1038/s41375-019-0522-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-019-0522-9
This article is cited by
-
The regulations of telomerase reverse transcriptase (TERT) in cancer
Cell Death & Disease (2024)