Abstract
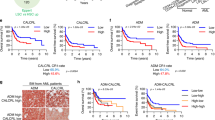
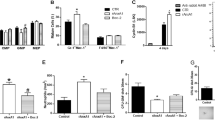
Calcitonin receptor-like (CALCRL) is a G-protein-coupled neuropeptide receptor involved in the regulation of blood pressure, angiogenesis, cell proliferation, and apoptosis, and is currently emerging as a novel target for the treatment of migraine. This study characterizes the role of CALCRL in acute myeloid leukemia (AML). We analyzed CALCRL expression in collectively more than 1500 well-characterized AML patients from five international cohorts (AMLCG, HOVON, TCGA, Leucegene, and UKM) and evaluated associations with survival. In the AMLCG analytic cohort, increasing transcript levels of CALCRL were associated with decreasing complete remission rates (71.5%, 53.7%, 49.6% for low, intermediate, high CALCRL expression), 5-year overall (43.1%, 26.2%, 7.1%), and event-free survival (29.9%, 15.8%, 4.7%) (all P < 0.001). CALCRL levels remained associated with all endpoints on multivariable regression analyses. The prognostic impact was confirmed in all validation sets. Genes highly expressed in CALCRLhigh AML were significantly enriched in leukemic stem cell signatures and CALCRL levels were positively linked to the engraftment capacity of primary patient samples in immunocompromised mice. CRISPR-Cas9-mediated knockout of CALCRL significantly impaired colony formation in human myeloid leukemia cell lines. Overall, our study demonstrates that CALCRL predicts outcome beyond existing risk factors and is a potential therapeutic target in AML.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, Matsuo H, et al. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun. 1993;192:553–60.
Brain SD, Williams TJ, Tippins JR, Morris HR, MacIntyre I. Calcitonin gene-related peptide is a potent vasodilator. Nature. 1985;313:54–6.
Larrayoz IM, Martinez-Herrero S, Garcia-Sanmartin J, Ochoa-Callejero L, Martinez A. Adrenomedullin and tumour microenvironment. J Transl Med. 2014;12:339.
Russell FA, King R, Smillie SJ, Kodji X, Brain SD. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev. 2014;94:1099–142.
Davis RB, Kechele DO, Blakeney ES, Pawlak JB, Caron KM. Lymphatic deletion of calcitonin receptor-like receptor exacerbates intestinal inflammation. JCI Insight. 2017;2:e92465.
Hershey AD. CGRP—the next frontier for migraine. N Engl J Med. 2017;377:2190–1.
Dodick DW Migraine. Lancet 2018;391:1315–30.
Berenguer-Daize C, Boudouresque F, Bastide C, Tounsi A, Benyahia Z, Acunzo J, et al. Adrenomedullin blockade suppresses growth of human hormone-independent prostate tumor xenograft in mice. Clin Cancer Res. 2013;19:6138–50.
Kaafarani I, Fernandez-Sauze S, Berenguer C, Chinot O, Delfino C, Dussert C, et al. Targeting adrenomedullin receptors with systemic delivery of neutralizing antibodies inhibits tumor angiogenesis and suppresses growth of human tumor xenografts in mice. FASEB J. 2009;23:3424–35.
Ouafik L, Sauze S, Boudouresque F, Chinot O, Delfino C, Fina F, et al. Neutralization of adrenomedullin inhibits the growth of human glioblastoma cell lines in vitro and suppresses tumor xenograft growth in vivo. Am J Pathol. 2002;160:1279–92.
Chen P, Huang Y, Bong R, Ding Y, Song N, Wang X, et al. Tumor-associated macrophages promote angiogenesis and melanoma growth via adrenomedullin in a paracrine and autocrine manner. Clin Cancer Res. 2011;17:7230–9.
Toda M, Suzuki T, Hosono K, Hayashi I, Hashiba S, Onuma Y, et al. Neuronal system-dependent facilitation of tumor angiogenesis and tumor growth by calcitonin gene-related peptide. Proc Natl Acad Sci USA. 2008;105:13550–5.
Harzenetter MD, Keller U, Beer S, Riedl C, Peschel C, Holzmann B. Regulation and function of the CGRP receptor complex in human granulopoiesis. Exp Hematol. 2002;30:306–12.
Chute JP, Muramoto GG, Dressman HK, Wolfe G, Chao NJ, Lin S. Molecular profile and partial functional analysis of novel endothelial cell-derived growth factors that regulate hematopoiesis. Stem Cells. 2006;24:1315–27.
Broome CS, Whetton AD, Miyan JA. Neuropeptide control of bone marrow neutrophil production is mediated by both direct and indirect effects on CFU-GM. Br J Haematol. 2000;108:140–50.
De Angeli S, Di Liddo R, Buoro S, Toniolo L, Conconi MT, Belloni AS, et al. New immortalized human stromal cell lines enhancing in vitro expansion of cord blood hematopoietic stem cells. Int J Mol Med. 2004;13:363–71.
Krug U, Berdel WE, Gale RP, Haferlach C, Schnittger S, Muller-Tidow C, et al. Increasing intensity of therapies assigned at diagnosis does not improve survival of adults with acute myeloid leukemia. Leukemia. 2016;30:1230–6.
Verhaak RG, Wouters BJ, Erpelinck CA, Abbas S, Beverloo HB, Lugthart S, et al. Prediction of molecular subtypes in acute myeloid leukemia based on gene expression profiling. Haematologica. 2009;94:131–4.
Valk PJ, Verhaak RG, Beijen MA, Erpelinck CA, Barjesteh van Waalwijk van Doorn-Khosrovani S, Boer JM, et al. Prognostically useful gene-expression profiles in acute myeloid leukemia. N Engl J Med. 2004;350:1617–28.
The Cancer Genome Atlas Research Network. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368:2059–74.
Lavallee VP, Baccelli I, Krosl J, Wilhelm B, Barabe F, Gendron P, et al. The transcriptomic landscape and directed chemical interrogation of MLL-rearranged acute myeloid leukemias. Nat Genet. 2015;47:1030–7.
Herold T, Metzeler KH, Vosberg S, Hartmann L, Rollig C, Stolzel F, et al. Isolated trisomy 13 defines a homogeneous AML subgroup with high frequency of mutations in spliceosome genes and poor prognosis. Blood. 2014;124:1304–11.
Schuffler PJ, Fuchs TJ, Ong CS, Wild PJ, Rupp NJ, Buhmann JM. TMARKER: a free software toolkit for histopathological cell counting and staining estimation. J Pathol Inf. 2013;4(Suppl):S2.
Pabst C, Bergeron A, Lavallee VP, Yeh J, Gendron P, Norddahl GL, et al. GPR56 identifies primary human acute myeloid leukemia cells with high repopulating potential in vivo. Blood. 2016;127:2018–27.
Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–47.
Zou H, Hastie T. Regularization and variable selection via the elastic net. J R Stat SocSer B (Stat Methodol). 2005;67:301–20.
Langer C, Marcucci G, Holland KB, Radmacher MD, Maharry K, Paschka P, et al. Prognostic importance of MN1 transcript levels, and biologic insights from MN1-associated gene and microRNA expression signatures in cytogenetically normal acute myeloid leukemia: a cancer and leukemia group B study. J Clin Oncol. 2009;27:3198–204.
Langer C, Radmacher MD, Ruppert AS, Whitman SP, Paschka P, Mrozek K, et al. High BAALC expression associates with other molecular prognostic markers, poor outcome, and a distinct gene-expression signature in cytogenetically normal patients younger than 60 years with acute myeloid leukemia: a Cancer and Leukemia Group B (CALGB) study. Blood. 2008;111:5371–9.
Schwind S, Marcucci G, Kohlschmidt J, Radmacher MD, Mrozek K, Maharry K, et al. Low expression of MN1 associates with better treatment response in older patients with de novo cytogenetically normal acute myeloid leukemia. Blood. 2011;118:4188–98.
Tanner SM, Austin JL, Leone G, Rush LJ, Plass C, Heinonen K, et al. BAALC, the human member of a novel mammalian neuroectoderm gene lineage, is implicated in hematopoiesis and acute leukemia. Proc Natl Acad Sci USA. 2001;98:13901–6.
Baldus CD, Thiede C, Soucek S, Bloomfield CD, Thiel E, Ehninger G. BAALC expression and FLT3 internal tandem duplication mutations in acute myeloid leukemia patients with normal cytogenetics: prognostic implications. J Clin Oncol. 2006;24:790–7.
Heuser M, Beutel G, Krauter J, Dohner K, von Neuhoff N, Schlegelberger B, et al. High meningioma 1 (MN1) expression as a predictor for poor outcome in acute myeloid leukemia with normal cytogenetics. Blood. 2006;108:3898–905.
Hattori A, Tsunoda M, Konuma T, Kobayashi M, Nagy T, Glushka J, et al. Cancer progression by reprogrammed BCAA metabolism in myeloid leukaemia. Nature. 2017;545:500–4.
Garrison BS, Rybak AP, Beerman I, Heesters B, Mercier FE, Scadden DT, et al. ZFP521 regulates murine hematopoietic stem cell function and facilitates MLL-AF9 leukemogenesis in mouse and human cells. Blood. 2017;130:619–24.
Ng SW, Mitchell A, Kennedy JA, Chen WC, McLeod J, Ibrahimova N, et al. A 17-gene stemness score for rapid determination of risk in acute leukaemia. Nature. 2016;540:433–7.
Stelljes M, Krug U, Beelen DW, Braess J, Sauerland MC, Heinecke A, et al. Allogeneic transplantation versus chemotherapy as postremission therapy for acute myeloid leukemia: a prospective matched pairs analysis. J Clin Oncol. 2014;32:288–96.
Marino R, Struck J, Maisel AS, Magrini L, Bergmann A, Di Somma S. Plasma adrenomedullin is associated with short-term mortality and vasopressor requirement in patients admitted with sepsis. Crit Care. 2014;18:R34.
Koyama T, Ochoa-Callejero L, Sakurai T, Kamiyoshi A, Ichikawa-Shindo Y, Iinuma N, et al. Vascular endothelial adrenomedullin-RAMP2 system is essential for vascular integrity and organ homeostasis. Circulation. 2013;127:842–53.
Nikitenko LL, Leek R, Henderson S, Pillay N, Turley H, Generali D, et al. The G-protein-coupled receptor CLR is upregulated in an autocrine loop with adrenomedullin in clear cell renal cell carcinoma and associated with poor prognosis. Clin Cancer Res. 2013;19:5740–8.
Martinez A, Vos M, Guedez L, Kaur G, Chen Z, Garayoa M, et al. The effects of adrenomedullin overexpression in breast tumor cells. J Natl Cancer Inst. 2002;94:1226–37.
Oehler MK, Hague S, Rees MC, Bicknell R. Adrenomedullin promotes formation of xenografted endometrial tumors by stimulation of autocrine growth and angiogenesis. Oncogene. 2002;21:2815–21.
Dong J, He Y, Zhang X, Wang L, Sun T, Zhang M, et al. Calcitonin gene-related peptide regulates the growth of epidermal stem cells in vitro. Peptides. 2010;31:1860–5.
Martinez-Herrero S, Larrayoz IM, Ochoa-Callejero L, Garcia-Sanmartin J, Martinez A. Adrenomedullin as a growth and cell fate regulatory factor for adult neural stem cells. Stem Cells Int. 2012;2012:804717.
Padro T, Ruiz S, Bieker R, Burger H, Steins M, Kienast J, et al. Increased angiogenesis in the bone marrow of patients with acute myeloid leukemia. Blood. 2000;95:2637–44.
Passaro D, Di Tullio A, Abarrategi A, Rouault-Pierre K, Foster K, Ariza-McNaughton L, et al. Increased vascular permeability in the bone marrow microenvironment contributes to disease progression and drug response in acute myeloid leukemia. Cancer Cell. 2017;32:324–41. e326.
Schepers K, Campbell TB, Passegue E. Normal and leukemic stem cell niches: insights and therapeutic opportunities. Cell Stem Cell. 2015;16:254–67.
Schliemann C, Bieker R, Padro T, Kessler T, Hintelmann H, Buchner T, et al. Expression of angiopoietins and their receptor Tie2 in the bone marrow of patients with acute myeloid leukemia. Haematologica. 2006;91:1203–11.
Dias S, Hattori K, Zhu Z, Heissig B, Choy M, Lane W, et al. Autocrine stimulation of VEGFR-2 activates human leukemic cell growth and migration. J Clin Invest. 2000;106:511–21.
Papaioannou D, Shen C, Nicolet D, McNeil B, Bill M, Karunasiri M, et al. Prognostic and biological significance of the proangiogenic factor EGFL7 in acute myeloid leukemia. Proc Natl Acad Sci USA. 2017;114:e4641–7.
Di Liddo R, Bridi D, Gottardi M, De Angeli S, Grandi C, Tasso A, et al. Adrenomedullin in the growth modulation and differentiation of acute myeloid leukemia cells. Int J Oncol. 2016;48:1659–69.
Kocemba KA, van Andel H, de Haan-Kramer A, Mahtouk K, Versteeg R, Kersten MJ, et al. The hypoxia target adrenomedullin is aberrantly expressed in multiple myeloma and promotes angiogenesis. Leukemia. 2013;27:1729–37.
Reuter U, Goadsby PJ, Lanteri-Minet M, Wen SH, Hours-Zesiger P, Ferrari MD, et al. Efficacy and tolerability of erenumab in patients with episodic migraine in whom two-to-four previous preventive treatments were unsuccessful: a randomised, double-blind, placebo-controlled, phase 3b study. Lancet. 2018;392:2280–7.
Acknowledgements
This work is dedicated to the memory of Professor Thomas Büchner. We thank all patients and clinicians participating in the trials. The help of Irina Arnhold, Mirco Witte, and Hans-Joachim Schnittler with TMA staining and analysis is gratefully acknowledged. The statistical analysis was partially funded by the José Carreras Foundation (DJCLS H 09/01f to DG). The Leucegene project is supported by the Government of Canada through Genome Canada and the Ministère de l’économie, de l’innovation et des exportations du Québec through Génome Québec, with supplementary funds from AmorChem. GS and JH are recipients of research chairs from the Canada Research Chair program and Industrielle-Alliance (Université de Montréal), respectively. The Banque de cellules leucémiques du Québec (BCLQ) is supported by grants from the Cancer Research Network of the Fonds de recherche du Québec-Santé. CP is supported by the German Cancer Aid (70111531). SKB is supported by Leukemia & Blood Cancer New Zealand and the family of Marijanna Kumerich. GL and WEB are supported by the German Research Foundation (DFG EXC 1003, Cluster of excellence “Cells in Motion”). TH and KS are supported by the Wilhelm Sander Foundation (2013.086.1 to TH and KS and 2013.086.2 to TH). TH is supported by the Physician Scientists Grant (G-509200-004) from the Helmholtz Zentrum München. C Schliemann and LA are supported by the Innovative Medical Research Fund of the University of Münster Medical School (SC211008 to CS and AN111813 to LA).
Author information
Authors and Affiliations
Contributions
LA, EB, TH and C Schliemann designed the study. LA, MCS, DG, AA, UK, KHM, C Schliemann, and EB performed statistical studies and analyzed the data. KW, TK, RMM, MS, HS and GL contributed to data analysis. KHM, SKB, MR-T, KS, W Hiddemann, and TH provided expression and mutational data on the AMLCG cohort. MCS, BJW, W Hiddemann, and WEB coordinated the AMLCG99 trial. JH, GS, PJMV and BL provided expression data and clinical annotations for validation cohorts. LA, LB, KD, C Schwöppe, W Hartmann, and C Schliemann characterized the UKM cohort and performed TMA stainings. CP and CMT provided expression data in hematopoietic subsets and in vivo engraftment experiments. VA, MFA and J-HM performed the CRISPR-Cas9 experiments. C Schliemann and LA wrote the manuscript. All authors interpreted the data and made the decision to submit the manuscript for publication.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Angenendt, L., Bormann, E., Pabst, C. et al. The neuropeptide receptor calcitonin receptor-like (CALCRL) is a potential therapeutic target in acute myeloid leukemia. Leukemia 33, 2830–2841 (2019). https://doi.org/10.1038/s41375-019-0505-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-019-0505-x
This article is cited by
-
Crosstalk Between Peripheral Innervation and Pancreatic Ductal Adenocarcinoma
Neuroscience Bulletin (2023)
-
Identification of significant genes as prognostic markers and potential tumor suppressors in lung adenocarcinoma via bioinformatical analysis
BMC Cancer (2021)
-
The neuropeptide calcitonin gene-related peptide links perineural invasion with lymph node metastasis in oral squamous cell carcinoma
BMC Cancer (2021)
-
Identifying a novel 5-gene signature predicting clinical outcomes in acute myeloid leukemia
Clinical and Translational Oncology (2021)
-
Adrenomedullin-CALCRL axis controls relapse-initiating drug tolerant acute myeloid leukemia cells
Nature Communications (2021)