Abstract
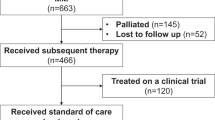
The introduction of CD38-targeting monoclonal antibodies (CD38 MoABs), daratumumab and isatuximab, has significantly impacted the management of patients with multiple myeloma (MM). Outcomes of patients with MM refractory to CD38 MoABs have not been described. We analyzed outcomes of 275 MM patients at 14 academic centers with disease refractory to CD38 MoABs. Median interval between MM diagnosis and refractoriness to CD38 MoAB (T0) was 50.1 months. The median overall survival (OS) from T0 for the entire cohort was 8.6 [95% C.I. 7.5–9.9] months, ranging from 11.2 months for patients not simultaneously refractory to an immunomodulatory (IMiD) agent and a proteasome inhibitor (PI) to 5.6 months for “penta-refractory” patients (refractory to CD38 MoAB, 2 PIs and 2 IMiDs). At least one subsequent treatment regimen was employed after T0 in 249 (90%) patients. Overall response rate to first regimen after T0 was 31% with median progression-free survival (PFS) and OS of 3.4 and 9.3 months, respectively. PFS was best achieved with combinations of carfilzomib and alkylator (median 5.7 months), and daratumumab and IMiD (median 4.5 months). Patients with MM refractory to CD38 MoAB have poor prognosis and this study provides benchmark for new therapies to be tested in this population.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Moreau P, Richardson PG, Cavo M, Orlowski RZ, San Miguel JF, Palumbo A, et al. Proteasome inhibitors in multiple myeloma: 10 years later. Blood. 2012;120:947–59.
Kumar SK, Dispenzieri A, Lacy MQ, Gertz MA, Buadi FK, Pandey S, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014;28:1122–8.
Moreau P. How I treat myeloma with new agents. Blood. 2017;130:1507–13.
Rajkumar SV, Kumar S. Multiple myeloma: diagnosis and treatment. Mayo Clin Proc. 2016;91:101–19.
Kumar SK, Dimopoulos MA, Kastritis E, Terpos E, Nahi H, Goldschmidt H, et al. Natural history of relapsed myeloma, refractory to immunomodulatory drugs and proteasome inhibitors: a multicenter IMWG study. Leukemia. 2017;31:2443–8.
Van de Donk NWCJ, Richardson PG, Malavasi F. CD38antibodies in multiple myeloma: back to the future. Blood. 2018;131:13–29.
Richter JR, Martin TG, Vij R, Cole C, Atanackovic D, Zonder JA, et al. Updated data from a phase II dose finding trial of single agent isatuximab (SAR650984, anti-CD38 mAb) in relapsed/refractory multiple myeloma (RRMM). J Clin Oncol. 2016;34 15_suppl:8005–8005.
Martin T, Baz R, Benson DM, Lendvai N, Wolf J, Munster P, et al. A phase 1b study of isatuximab plus lenalidomide and dexamethasone for relapsed/refractory multiple myeloma. Blood. 2017;129:3294–303.
Martin TG, Mannis GN, Chari A, Munster P, Campana F, Hui A-M, et al. Phase Ib study of isatuximab and carfilzomib in relapse and refractory multiple myeloma. Blood. 2016;128:2111–2111.
Mikhael J, Richardson PG, Usmani SZ, Raje NS, Bensinger W, Dubin F, et al. Final results of a phase Ib study of isatuximab (ISA) plus pomalidomide (Pom) and dexamethasone (dex) in relapsed/refractory multiple myeloma (RRMM). J Clin Oncol. 2018;36 15_suppl:8038–8038.
Lonial S, Weiss BM, Usmani SZ, Singhal S, Chari A, Bahlis NJ, et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet (Lond, Engl). 2016;387:1551–60.
Chari A, Suvannasankha A, Fay JW, Arnulf B, Kaufman JL, Ifthikharuddin JJ, et al. Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood. 2017;130:974–81.
Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis NJ, Usmani SZ, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:1319–31.
Palumbo A, Chanan-Khan A, Weisel K, Nooka AK, Masszi T, Beksac M, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:754–66.
Durie BGM, Harousseau JL, Miguel JS, Bladé J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467. 07/20/online
Rajkumar SV, Harousseau J-L, Durie B, Anderson KC, Dimopoulos M, Kyle R, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117:4691–5.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81.
Nooka AK, Joseph N, Boise LH, Gleason C, Kaufman JL, Lonial S. Clinical efficacy of daratumumab, pomalidomide and dexamethasone in relapsed, refractory myeloma patients: utility of retreatment with daratumumab among refractory patients. Blood. 2016;128:492–492.
Chari A, Suvannasankha A, Fay JW, Arnulf B, Kaufman JL, Ifthikharuddin JJ, et al. Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood. 2017;130:974–81.
Kumar SK, Kapoor P, Laplant B, Muchtar E, Buadi FK, Gonsalves WI, et al. Phase 2 Trial of ixazomib, lenalidomide, dexamethasone and daratumumab in patients with newly diagnosed multiple myeloma. Blood. 2018;132 Suppl 1:304–304.
Facon T, Kumar SK, Plesner T, Orlowski RZ, Moreau P, Bahlis N, et al. Phase 3 randomized study of daratumumab plus lenalidomide and dexamethasone (D-Rd) versus lenalidomide and dexamethasone (Rd) in patients with newly diagnosed multiple myeloma (NDMM) ineligible for transplant (MAIA). Blood. 2018;132 Suppl 1:LBA-2–LBA-2.
Mateos MV, Dimopoulos MA, Cavo M, Suzuki K, Jakubowiak A, Knop S, et al. Daratumumab plus Bortezomib, Melphalan, and Prednisone for Untreated Myeloma. N Engl J Med. 2018;378:518–28.
Raje NS, Berdeja J, Lin Y, Munshi NC, Spigel D, Liedtke M, et al. bb2121 anti-BCMA CAR T-cell therapy in patients with relapsed/refractory multiple myeloma: updated results from a multicenter phase I study. J Clin Oncol. 2018:36:8007. https://doi.org/10.1200/JCO.2018.36.15_suppl.8007
Topp MS, Duell J, Zugmaier G, Attal M, Moreau P, Langer C, et al. Treatment with AMG 420, an anti-B-cell maturation antigen (BCMA) bispecific T-cell engager (BiTE®) antibody construct, induces minimal residual disease (MRD) negative complete responses in relapsed and/or refractory (R/R) multiple myeloma (MM) patients: results of a first-in-human (FIH) phase I dose escalation study. Blood. 2018;132 Suppl 1:1010–1010.
Trudel S, Lendvai N, Popat R, Voorhees PM, Reeves B, Libby EN, et al. Targeting B-cell maturation antigen with GSK2857916 antibody-drug conjugate in relapsed or refractory multiple myeloma (BMA117159): a dose escalation and expansion phase 1 trial. Lancet Oncol. 2018;19:1641–53.
Acknowledgements
We are thankful to the Institutional Review Boards and hospital staff at each participating academic center, REDCap IT support at Vanderbilt University Medical Center, and all the patients and their families. This study was not funded commercially.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
EhM: Consultancy and Speakers Bureau (Takeda, Celgene, Amgen, Janssen); BP: Stock and pension plan (Bristol Myer Squibb); AN: Consultancy (Celgene, Amgen); ML: Consultancy (Amgen/Onyx), Honoraria (Amgen/Onyx, Pfizer, Prothena, Takeda), Research Funding (Amgen/Onyx, BlueBirdBio, Celgene, Genentech/Roche, Gilead, Pfizer, Prothena, Takeda), Membership on an entity’s Board of Directors or advisory committees (Caelum, Pfizer, Prothena, Takeda); PH: Consultancy and Research Funding (Amgen, Celgene), Honoraria (Celgene); RV: Honoraria (Celgene, Bristol Myers Squibb, Takeda, Amgen, Janssen, Karyopharma, Jazz), Research Funding (Celgene, Bristol Myers Squibb, Takeda); SU: Consultancy (Abbvie, Amgen, Celgene, Genmab, Merck, MundiPharma, Seattle Genetics), Research Funding (Amgen, BMS, Celgene, Janssen, Merck, Pharmacyclics, Takeda), Membership on an entity’s Board of Directors or advisory committees (Sanofi); ShK: Consultancy (AbbVie, Celgene, Janssen, Kite Pharma, Merck, Takeda); LC: Honoraria (Amgen, Celgene, AbbVie), Research Funding (Amgen, Celgene, Janssen). The remaining authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gandhi, U.H., Cornell, R.F., Lakshman, A. et al. Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia 33, 2266–2275 (2019). https://doi.org/10.1038/s41375-019-0435-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-019-0435-7
This article is cited by
-
Challenges and strategies associated with CAR-T cell therapy in blood malignancies
Experimental Hematology & Oncology (2024)
-
Efficacy and safety of bispecific antibodies vs. immune checkpoint blockade combination therapy in cancer: a real-world comparison
Molecular Cancer (2024)
-
Efficacy and immune modulation associated with the addition of IMiDs to Daratumumab backbone in multiple myeloma patients refractory to both drug classes: resetting synergistic activity
Blood Cancer Journal (2024)
-
Teclistamab in relapsed refractory multiple myeloma: multi-institutional real-world study
Blood Cancer Journal (2024)
-
Bispecific CAR T cell therapy targeting BCMA and CD19 in relapsed/refractory multiple myeloma: a phase I/II trial
Nature Communications (2024)