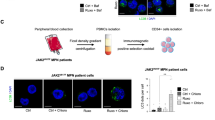
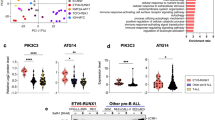
Abstract
Chaperone-mediated autophagy (CMA) is a highly selective form of autophagy. During CMA, the HSC70 chaperone carries target proteins endowed with a KFERQ-like motif to the lysosomal receptor LAMP2A, which then translocate them into lysosomes for degradation. In the present study, we scrutinized the mechanisms underlying the response and resistance to Azacytidine (Aza) in MDS/AML cell lines and bone marrow CD34+ blasts from MDS/AML patients. In engineered Aza-resistant MDS cell lines and some AML cell lines, we identified a profound defect in CMA linked to the absence of LAMP2A. LAMP2 deficiency was responsible for Aza resistance and hypersensitivity to lysosome and autophagy inhibitors. Accordingly, gain of function of LAMP2 in deficient cells or loss of function in LAMP2-expressing cells rendered them sensitive or resistant to Aza, respectively. A strict correlation was observed between the absence of LAMP2, resistance to Aza and sensitivity to lysosome inhibitors. Low levels of LAMP2 expression in CD34+ blasts from MDS/AML patients correlated with lack of sensitivity to Aza and were predictive of poor overall survival. We propose that CD34+/LAMP2Low patients at diagnosis or who become CD34+/LAMP2Low during the course of treatment with Aza might benefit from a lysosome inhibitor already used in the clinic.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Change history
14 July 2020
A Correction to this paper has been published: https://doi.org/10.1038/s41375-020-0969-8
References
Tefferi A, Vardiman JW. Myelodysplastic syndromes. N Engl J Med. 2009;361:1872–85.
Fenaux P. Myelodysplastic syndromes: from pathogenesis and prognosis to treatment. Semin Hematol. 2004;41(2Suppl 4):6–12.
Mufti G, List AF, Gore SD, Ho AY. Myelodysplastic syndrome. Hematology Am Soc Hematol Educ Program. 2003;1:176–99.
Mufti GJ. Pathobiology, classification, and diagnosis of myelodysplastic syndrome. Best Pract Res Clin Haematol. 2004;17:543–57.
Gardin C, Chaibi P, de Revel T, Rousselot P, Turlure P, Miclea JM, et al. Intensive chemotherapy with idarubicin, cytosine arabinoside, and granulocyte colony-stimulating factor (G-CSF) in patients with secondary and therapy-related acute myelogenous leukemia. Club de Reflexion en Hematologie. Leukemia. 1997;11:16–21.
Ades L, Santini V. Hypomethylating agents and chemotherapy in MDS. Best Pract Res Clin Haematol. 2013;26:411–9.
Kantarjian HM, Thomas XG, Dmoszynska A, Wierzbowska A, Mazur G, Mayer J, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30:2670–7.
Itzykson R, Fenaux P. Epigenetics of myelodysplastic syndromes. Leukemia. 2014;28:497–506.
Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–32.
Silverman LR, Demakos EP, Peterson BL, Kornblith AB, Holland JC, Odchimar-Reissig R, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002;20:2429–40.
Santini V, Prebet T, Fenaux P, Gattermann N, Nilsson L, Pfeilstocker M, et al. Minimizing risk of hypomethylating agent failure in patients with higher-risk MDS and practical management recommendations. Leuk Res. 2014;38:1381–91.
Prebet T, Gore SD, Esterni B, Gardin C, Itzykson R, Thepot S, et al. Outcome of high-risk myelodysplastic syndrome after azacitidine treatment failure. J Clin Oncol. 2011;29:3322–7.
Petersen NH, Olsen OD, Groth-Pedersen L, Ellegaard AM, Bilgin M, Redmer S, et al. Transformation-associated changes in sphingolipid metabolism sensitize cells to lysosomal cell death induced by inhibitors of acid sphingomyelinase. Cancer Cell. 2013;24:379–93.
Piao S, Amaravadi RK. Targeting the lysosome in cancer. Ann N Y Acad Sci. 2016;1371:45–54.
Kallunki T, Olsen OD, Jaattela M. Cancer-associated lysosomal changes: friends or foes? Oncogene. 2013;32:1995–2004.
Cluzeau T, Robert G, Puissant A, Jean-Michel K, Cassuto JP, Raynaud S, et al. Azacitidine-resistant SKM1 myeloid cells are defective for AZA-induced mitochondrial apoptosis and autophagy. Cell Cycle. 2011;10:2339–43.
Cluzeau T, Dubois A, Jacquel A, Luciano F, Renneville A, Preudhomme C, et al. Phenotypic and genotypic characterization of azacitidine-sensitive and resistant SKM1 myeloid cell lines. Oncotarget. 2014;5:4384–91.
Guo S, Liang Y, Murphy SF, Huang A, Shen H, Kelly DF, et al. A rapid and high content assay that measures cyto-ID-stained autophagic compartments and estimates autophagy flux with potential clinical applications. Autophagy. 2015;11:560–72.
Li P, Ji M, Lu F, Zhang J, Li H, Cui T, et al. Degradation of AF1Q by chaperone-mediated autophagy. Exp Cell Res. 2014;327:48–56.
Massey A, Kiffin R, Cuervo AM. Pathophysiology of chaperone-mediated autophagy. Int J Biochem Cell Biol. 2004;36:2420–34.
Kaushik S, Massey AC, Mizushima N, Cuervo AM. Constitutive activation of chaperone-mediated autophagy in cells with impaired macroautophagy. Mol Biol Cell. 2008;19:2179–92.
Kaushik S, Cuervo AM. Chaperone-mediated autophagy: a unique way to enter the lysosome world. Trends Cell Biol. 2012;22:407–17.
Orenstein SJ, Cuervo AM. Chaperone-mediated autophagy: molecular mechanisms and physiological relevance. Semin Cell Dev Biol. 2010;21:719–26.
Dice JF. Chaperone-mediated autophagy. Autophagy. 2007;3:295–9.
Massey AC, Kaushik S, Sovak G, Kiffin R, Cuervo AM. Consequences of the selective blockage of chaperone-mediated autophagy. Proc Natl Acad Sci USA. 2006;103:5805–10.
Koga H, Martinez-Vicente M, Arias E, Kaushik S, Sulzer D, Cuervo AM. Constitutive upregulation of chaperone-mediated autophagy in Huntington’s disease. J Neurosci. 2011;31:18492–505.
Wu H, Chen S, Ammar AB, Xu J, Wu Q, Pan K, et al. Crosstalk between macroautophagy and chaperone-mediated autophagy: implications for the treatment of neurological diseases. Mol Neurobiol. 2015;52:1284–96.
Cai Z, Zeng W, Tao K, E Z, Wang B, Yang Q. Chaperone-mediated autophagy: roles in neuroprotection. Neurosci Bull. 2015;31:452–8.
Koga H, Cuervo AM. Chaperone-mediated autophagy dysfunction in the pathogenesis of neurodegeneration. Neurobiol Dis. 2011;43:29–37.
Park C, Cuervo AM. Selective autophagy: talking with the UPS. Cell Biochem Biophys. 2013;67:3–13.
Benbrook DM, Long A. Integration of autophagy, proteasomal degradation, unfolded protein response and apoptosis. Exp Oncol. 2012;34:286–97.
Vilchez D, Saez I, Dillin A. The role of protein clearance mechanisms in organismal ageing and age-related diseases. Nat Commun. 2014;5:5659.
Wojcik S. Crosstalk between autophagy and proteasome protein degradation systems: possible implications for cancer therapy. Folia Histochem Cytobiol. 2013;51:249–64.
Nedelsky NB, Todd PK, Taylor JP. Autophagy and the ubiquitin-proteasome system: collaborators in neuroprotection. Biochim Biophys Acta. 2008;1782:691–9.
Strunk CJ, Platzbecker U, Thiede C, Schaich M, Illmer T, Kang Z, et al. Elevated AF1q expression is a poor prognostic marker for adult acute myeloid leukemia patients with normal cytogenetics. Am J Hematol. 2009;84:308–9.
Cluzeau T, Robert G, Mounier N, Karsenti JM, Dufies M, Puissant A, et al. BCL2L10 is a predictive factor for resistance to azacitidine in MDS and AML patients. Oncotarget. 2012;3:490–501.
Hamouda MA, Jacquel A, Robert G, Puissant A, Richez V, Cassel R, et al. BCL-B (BCL2L10) is overexpressed in patients suffering from multiple myeloma (MM) and drives an MM-like disease in transgenic mice. J Exp Med. 2016;213:1705–22.
Saftig P, Tanaka Y, Lullmann-Rauch R, von Figura K. Disease model: LAMP-2 enlightens Danon disease. Trends Mol Med. 2001;7:37–39.
Rowland TJ, Sweet ME, Mestroni L, Taylor MR. Danon disease - dysregulation of autophagy in a multisystem disorder with cardiomyopathy. J Cell Sci. 2016;129:2135–43.
Le Guyader-Peyrou S, Belot A, Maynadie M, Binder-Foucard F, Remontet L, Troussard X, et al. Cancer incidence in France over the 1980–2012 period: Hematological malignancies. Rev Epidemiol Sante Publique. 2016;64:103–12.
Cuervo AM, Dice JF. Age-related decline in chaperone-mediated autophagy. J Biol Chem. 2000;275:31505–13.
Huang J, Xu J, Pang S, Bai B, Yan B. Age-related decrease of the LAMP-2 gene expression in human leukocytes. Clin Biochem. 2012;45:1229–32.
Acknowledgements
This work was supported by INSERM, INCA (PRTK-045, 2013–2015), the Fondation ARC pour la Recherche contre le Cancer (Equipe labellisée 2014–2017 and 2018–2020) and the Association Laurette Fugain (ALF 2016/08). This work was also funded by the French government (National Research Agency, ANR) through the “investissement for the future” LABEX SIGNALIFE program reference #ANR-11-LABEX-0028-01. AD is the recipient of a fellowship from the Fondation pour la Recherche Médicale. SB is the recipient of a fellowship from the Fondation ARC. MZ is the recipient of a fellowship from the LABEX SIGNALIFE program. AP is supported by the ATIP-AVENIR research grant and by the St Louis Association for leukemia research. Finally, this work was also supported by the Conseil General des Alpes Maritimes and the Conseil Regional PACA & Corse. The authors greatly acknowledge the C3M Imaging Core Facility part of MICA (Microscopy and Imaging Platform Côte d’Azur).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
About this article
Cite this article
Dubois, A., Furstoss, N., Calleja, A. et al. RETRACTED ARTICLE: LAMP2 expression dictates azacytidine response and prognosis in MDS/AML. Leukemia 33, 1501–1513 (2019). https://doi.org/10.1038/s41375-018-0336-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-018-0336-1
This article is cited by
-
Epigenetic and post-translational modifications in autophagy: biological functions and therapeutic targets
Signal Transduction and Targeted Therapy (2023)
-
Retraction Note: LAMP2 expression dictates azacytidine response and prognosis in MDS/AML
Leukemia (2020)