Abstract
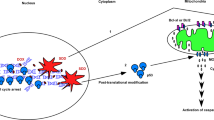
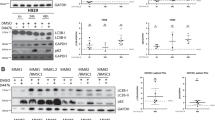
B7-H3 (CD276) is broadly overexpressed by multiple human cancers. It plays a vital role in tumor progression and has been accepted as one of the inhibitory B7 family checkpoint molecules. To identify the functions and underlying mechanisms of B7-H3 in multiple myeloma, we analyzed B7-H3 expression in myeloma patients and used siRNAs and overexpression plasmid of B7-H3 to investigate its roles and downstream signaling molecules in myeloma cell lines. The results showed that surface expression of B7-H3 was upregulated in myeloma samples and cell lines. Lower expression of B7-H3 in myeloma cells was associated with better progression-free survival. Myeloma cell survival, drug resistance, and tumor growth could be promoted by B7-H3. The molecular basis for these functional roles of B7-H3 involved the activation of JAK2/STAT3 via redox-mediated oxidation and activation of Src. We further identified a STAT3-promoting signaling pathway by which oxidant-mediated Src phosphorylation led to secondary activation of the E3 ubiquitin ligase c-Cbl. Activated c-Cbl subsequently caused specific proteasomal degradation of SOCS3, a negative regulator of JAK2/STAT3. These data indicate B7-H3’s important role in the activation of ROS/Src/c-Cbl pathway in multiple myeloma which integrates redox regulation and sustained STAT3 activation at the level of degradation of STAT3 suppressor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Rajkumar SV, Kumar S. Multiple myeloma: diagnosis and treatment. Mayo Clin Proc. 2016;91:101–19.
Gorgun G, Samur MK, Cowens KB, Paula S, Bianchi G, Anderson JE, et al. Lenalidomide enhances immune checkpoint blockade-induced immune response in multiple myeloma. Clin Cancer Res. 2015;21:4607–18.
Janakiram M, Pareek V, Cheng H, Narasimhulu DM, Zang X. Immune checkpoint blockade in human cancer therapy: lung cancer and hematologic malignancies. Immunotherapy. 2016;8:809–19.
Kuranda K, Berthon C, Dupont C, Wolowiec D, Leleu X, Polakowska R, et al. A subpopulation of malignant CD34+CD138+B7-H1+plasma cells is present in multiple myeloma patients. Exp Hematol. 2010;38:124–31.
Liu J, Hamrouni A, Wolowiec D, Coiteux V, Kuliczkowski K, Hetuin D, et al. Plasma cells from multiple myeloma patients express B7-H1 (PD-L1) and increase expression after stimulation with IFN-{gamma} and TLR ligands via a MyD88-, TRAF6-, and MEK-dependent pathway. Blood. 2007;110:296–304.
Yamashita T, Tamura H, Satoh C, Shinya E, Takahashi H, Chen L, et al. Functional B7.2 and B7-H2 molecules on myeloma cells are associated with a growth advantage. Clin Cancer Res. 2009;15:770–7.
Jeon YK, Park SG, Choi IW, Lee SW, Lee SM, Choi I. Cancer cell-associated cytoplasmic B7-H4 is induced by hypoxia through hypoxia-inducible factor-1alpha and promotes cancer cell proliferation. Biochem Biophys Res Commun. 2015;459:277–83.
Tamura H, Ishibashi M, Yamashita T, Tanosaki S, Okuyama N, Kondo A, et al. Marrow stromal cells induce B7-H1 expression on myeloma cells, generating aggressive characteristics in multiple myeloma. Leukemia. 2013;27:464–72.
Ni L, Dong C. New checkpoints in cancer immunotherapy. Immunol Rev. 2017;276:52–65.
Janakiram M, Shah UA, Liu W, Zhao A, Schoenberg MP, Zang X. The third group of the B7-CD28 immune checkpoint family: HHLA2, TMIGD2, B7x, and B7-H3. Immunol Rev. 2017;276:26–39.
Wu CP, Jiang JT, Tan M, Zhu YB, Ji M, Xu KF, et al. Relationship between co-stimulatory molecule B7-H3 expression and gastric carcinoma histology and prognosis. World J Gastroenterol. 2006;12:457–9.
Lee YH, Martin-Orozco N, Zheng P, Li J, Zhang P, Tan H, et al. Inhibition of the B7-H3 immune checkpoint limits tumor growth by enhancing cytotoxic lymphocyte function. Cell Res. 2017;27:1034–45.
Lim S, Liu H, Madeira da Silva L, Arora R, Liu Z, Phillips JB, et al. Immunoregulatory protein B7-H3 reprograms glucose metabolism in cancer cells by ROS-mediated stabilization of HIF1alpha. Cancer Res. 2016;76:2231–42.
Luo D, Xiao H, Dong J, Li Y, Feng G, Cui M, et al. B7-H3 regulates lipid metabolism of lung cancer through SREBP1-mediated expression of FASN. Biochem Biophys Res Commun. 2017;482:1246–51.
Xie C, Liu D, Chen Q, Yang C, Wang B, Wu H. Soluble B7-H3 promotes the invasion and metastasis of pancreatic carcinoma cells through the TLR4/NF-kappaB pathway. Sci Rep. 2016;6:27528.
Chen X, Meng X, Foley NM, Shi X, Liu M, Chai Y, et al. Activation of the TLR2-mediated downstream signaling pathways NF-kappaB and MAPK is responsible for B7-H3-augmented inflammatory response during S. pneumoniae infection. J Neuroimmunol. 2017;310:82–90.
Fauci JM, Sabbatino F, Wang Y, Londono-Joshi AI, Straughn JM Jr., Landen CN, et al. Monoclonal antibody-based immunotherapy of ovarian cancer: targeting ovarian cancer cells with the B7-H3-specific mAb 376.96. Gynecol Oncol. 2014;132:203–10.
Ahmed M, Cheng M, Zhao Q, Goldgur Y, Cheal SM, Guo HF, et al. Humanized affinity-matured monoclonal antibody 8H9 has potent antitumor activity and binds to FG loop of tumor antigen B7-H3. J Biol Chem. 2015;290:30018–29.
Kang FB, Wang L, Jia HC, Li D, Li HJ, Zhang YG, et al. B7-H3 promotes aggression and invasion of hepatocellular carcinoma by targeting epithelial-to-mesenchymal transition via JAK2/STAT3/Slug signaling pathway. Cancer Cell Int. 2015;15:45.
Loo D, Alderson RF, Chen FZ, Huang L, Zhang W, Gorlatov S, et al. Development of an Fc-enhanced anti-B7-H3 monoclonal antibody with potent antitumor activity. Clin Cancer Res. 2012;18:3834–45.
Ma J, Ma P, Zhao C, Xue X, Han H, Liu C, et al. B7-H3 as a promising target for cytotoxicity T cell in human cancer therapy. Oncotarget. 2016;7:29480–91.
Marin-Acevedo JA, Dholaria B, Soyano AE, Knutson KL, Chumsri S, Lou Y. Next generation of immune checkpoint therapy in cancer: new developments and challenges. J Hematol Oncol. 2018;11:39.
Seaman S, Zhu Z, Saha S, Zhang XM, Yang MY, Hilton MB, et al. Eradication of tumors through simultaneous ablation of CD276/B7-H3-positive tumor cells and tumor vasculature. Cancer Cell. 2017;31:501–15 e508.
Semenza GL. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J Clin Invest. 2013;123:3664–71.
Zhang T, Jiang B, Zou ST, Liu F, Hua D. Overexpression of B7-H3 augments anti-apoptosis of colorectal cancer cells by Jak2-STAT3. World J Gastroenterol. 2015;21:1804–13.
Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, et al. Stat3 as an oncogene. Cell. 1999;98:295–303.
Horne WC, Sanjay A, Bruzzaniti A, Baron R. The role(s) of Src kinase and Cbl proteins in the regulation of osteoclast differentiation and function. Immunol Rev. 2005;208:106–25.
Lin L, Yan F, Zhao D, Lv M, Liang X, Dai H, et al. Reelin promotes the adhesion and drug resistance of multiple myeloma cells via integrin beta1 signaling and STAT3. Oncotarget. 2016;7:9844–58.
Ray S, Lee C, Hou T, Bhakat KK, Brasier AR. Regulation of signal transducer and activator of transcription 3 enhanceosome formation by apurinic/apyrimidinic endonuclease 1 in hepatic acute phase response. Mol Endocrinol. 2010;24:391–401.
Ray S, Ju X, Sun H, Finnerty CC, Herndon DN, Brasier AR. The IL-6 trans-signaling-STAT3 pathway mediates ECM and cellular proliferation in fibroblasts from hypertrophic scar. J Invest Dermatol. 2013;133:1212–20.
Groner B. Determinants of the extent and duration of STAT3 signaling. JAKSTAT. 2012;1:211–5.
Borges S, Moudilou E, Vouyovitch C, Chiesa J, Lobie P, Mertani H, et al. Involvement of a JAK/STAT pathway inhibitor: cytokine inducible SH2 containing protein in breast cancer. Adv Exp Med Biol. 2008;617:321–9.
Giannoni E, Buricchi F, Raugei G, Ramponi G, Chiarugi P. Intracellular reactive oxygen species activate Src tyrosine kinase during cell adhesion and anchorage-dependent cell growth. Mol Cell Biol. 2005;25:6391–403.
Xu D, Rovira II, Finkel T. Oxidants painting the cysteine chapel: redox regulation of PTPs. Dev Cell. 2002;2:251–2.
Hou J, Cui A, Song P, Hua H, Luo T, Jiang Y. Reactive oxygen species-mediated activation of the Src-epidermal growth factor receptor-Akt signaling cascade prevents bortezomib-induced apoptosis in hepatocellular carcinoma cells. Mol Med Rep. 2015;11:712–8.
Ke K, Sul OJ, Choi EK, Safdar AM, Kim ES, Choi HS. Reactive oxygen species induce the association of SHP-1 with c-Src and the oxidation of both to enhance osteoclast survival. Am J Physiol Endocrinol Metab. 2014;307:E61–70.
Hao Q, Rutherford SA, Low B, Tang H. Suppression of the phosphorylation of receptor tyrosine phosphatase-alpha on the Src-independent site tyrosine 789 by reactive oxygen species. Mol Pharmacol. 2006;69:1938–44.
Giannoni E, Chiarugi P. Redox circuitries driving Src regulation. Antioxid Redox Signal. 2014;20:2011–25.
Giannoni E, Taddei ML, Chiarugi P. Src redox regulation: again in the front line. Free Radic Biol Med. 2010;49:516–27.
Dou H, Buetow L, Hock A, Sibbet GJ, Vousden KH, Huang DT. Structural basis for autoinhibition and phosphorylation-dependent activation of c-Cbl. Nat Struct Mol Biol. 2012;19:184–92.
Kobashigawa Y, Tomitaka A, Kumeta H, Noda NN, Yamaguchi M, Inagaki F. Autoinhibition and phosphorylation-induced activation mechanisms of human cancer and autoimmune disease-related E3 protein Cbl-b. Proc Natl Acad Sci USA. 2011;108:20579–84.
Andoniou CE, Thien CB, Langdon WY. Tumour induction by activated abl involves tyrosine phosphorylation of the product of the cbl oncogene. EMBO J. 1994;13:4515–23.
Swaminathan G, Feshchenko EA, Tsygankov AY. c-Cbl-facilitated cytoskeletal effects in v-Abl-transformed fibroblasts are regulated by membrane association of c-Cbl. Oncogene. 2007;26:4095–105.
Wang L, Kang FB, Shan BE. B7-H3-mediated tumor immunology: friend or foe? Int J Cancer. 2014;134:2764–71.
Reindl C, Quentmeier H, Petropoulos K, Greif PA, Benthaus T, Argiropoulos B, et al. CBL exon 8/9 mutants activate the FLT3 pathway and cluster in core binding factor/11q deletion acute myeloid leukemia/myelodysplastic syndrome subtypes. Clin Cancer Res. 2009;15:2238–47.
Sanada M, Suzuki T, Shih LY, Otsu M, Kato M, Yamazaki S, et al. Gain-of-function of mutated C-CBL tumour suppressor in myeloid neoplasms. Nature. 2009;460:904–8.
Sargin B, Choudhary C, Crosetto N, Schmidt MH, Grundler R, Rensinghoff M, et al. Flt3-dependent transformation by inactivating c-Cbl mutations in AML. Blood. 2007;110:1004–12.
Cooper JA, Kaneko T, Li SS. Cell regulation by phosphotyrosine-targeted ubiquitin ligases. Mol Cell Biol. 2015;35:1886–97.
Blesofsky WA, Mowen K, Arduini RM, Baker DP, Murphy MA, Bowtell DD, et al. Regulation of STAT protein synthesis by c-Cbl. Oncogene. 2001;20:7326–33.
Rorsman C, Tsioumpekou M, Heldin CH, Lennartsson J. The ubiquitin ligases c-Cbl and Cbl-b negatively regulate platelet-derived growth factor (PDGF) BB-induced chemotaxis by affecting PDGF receptor beta (PDGFRbeta) internalization and signaling. J Biol Chem. 2016;291:11608–18.
Wang L, Rudert WA, Loutaev I, Roginskaya V, Corey SJ. Repression of c-Cbl leads to enhanced G-CSF Jak-STAT signaling without increased cell proliferation. Oncogene. 2002;21:5346–55.
Noble M, Mayer-Proschel M, Li Z, Dong T, Cui W, Proschel C, et al. Redox biology in normal cells and cancer: restoring function of the redox/Fyn/c-Cbl pathway in cancer cells offers new approaches to cancer treatment. Free Radic Biol Med. 2015;79:300–23.
Acknowledgements
The authors wish to thank Prof. Yingyu Chen, Yanhui Yin, Hongquan Zhang, and Genze Shao from Peking University Health Science Center for suggestions, critical comments, and reagents. The human myeloma cell lines NCI-H929 (shown as H929) and U266 were kindly provided by Prof. Jian Hou from Shanghai Chang Zheng Hospital and Prof. Yu Zhang from Peking University Health Science Center.
Funding
This work was supported by grants from the National Key Research and Development Program of China 2017YFA0104500, National Natural Science Foundation of China (31270935, 81471525, and 31671244, Q.G., 81670192, and YXJL-2017-0163-0006, J.L.), the Foundation for Innovative Research Groups of the National Natural Science Foundation of China (81621001, Q.G.) and the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lin, L., Cao, L., Liu, Y. et al. B7-H3 promotes multiple myeloma cell survival and proliferation by ROS-dependent activation of Src/STAT3 and c-Cbl-mediated degradation of SOCS3. Leukemia 33, 1475–1486 (2019). https://doi.org/10.1038/s41375-018-0331-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-018-0331-6
This article is cited by
-
Dimerization of the 4Ig isoform of B7-H3 in tumor cells mediates enhanced proliferation and tumorigenic signaling
Communications Biology (2024)
-
Tumor-expressed B7-H3 promotes vasculogenic mimicry formation rather than angiogenesis in non-small cell lung cancer
Journal of Cancer Research and Clinical Oncology (2023)
-
B7-H3 is eligible for predicting clinical outcomes in lung adenocarcinoma patients treated with EGFR tyrosine kinase inhibitors
World Journal of Surgical Oncology (2022)
-
Reduced B7-H3 expression by PAX3-FOXO1 knockdown inhibits cellular motility and promotes myogenic differentiation in alveolar rhabdomyosarcoma
Scientific Reports (2021)
-
B7-H3 immune checkpoint expression is a poor prognostic factor in colorectal carcinoma
Modern Pathology (2020)