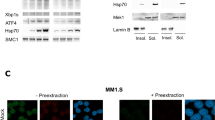
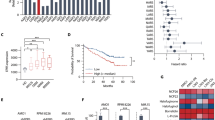
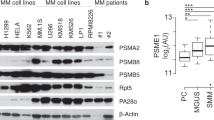
Abstract
Multiple Myeloma (MM) is highly sensitive to disruptions in cellular protein homeostasis. Proteasome inhibitors (PIs) are initially effective in the treatment of MM, although cures are not achievable and the emergence of resistance limits the durability of responses. New therapies are needed for refractory patients, and those that combat resistance to standard of care agents would be particularly valuable. Screening of multiple chemical libraries for PI re-sensitizing compounds identified E61 as a potent enhancer of multiple PIs and MM specific activity. Using a tandem approach of click chemistry and peptide mass fingerprinting, we identified multiple protein disulfide isomerase (PDI) family members as the primary molecular targets of E61. PDIs mediate oxidative protein folding, and E61 treatment induced robust ER and oxidative stress responses as well as the accumulation of ubiquitinylated proteins. A chemical optimization program led to a new structural class of indene (exemplified by lead E64FC26), which are highly potent pan-style inhibitors of PDIs. In mice with MM, E64FC26 improved survival and enhanced the activity of bortezomib without any adverse effects. This work demonstrates the potential of E64FC26 as an early drug candidate and the strategy of targeting multiple PDI isoforms for the treatment of refractory MM and beyond.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Shimizu Y, Hendershot LM. Oxidative folding: cellular strategies for dealing with the resultant equimolar production of reactive oxygen species. Antioxid Redox Signal. 2009;11:2317–31.
Brewer JW, Hendershot LM. Building an antibody factory: a job for the unfolded protein response. Nat Immunol. 2005;6:23–9.
Cenci S, Sitia R. Managing and exploiting stress in the antibody factory. FEBS Lett. 2007;581:3652–7.
Bertolotti M, Yim SH, Garcia-Manteiga JM, Masciarelli S, Kim YJ, Kang MH, et al. B- to plasma-cell terminal differentiation entails oxidative stress and profound reshaping of the antioxidant responses. Antioxid Redox Signal. 2010;13:1133–44.
Dolloff NG. Emerging therapeutic strategies for overcoming proteasome inhibitor resistance. Adv Cancer Res. 2015;127:191–226.
Boise LH, Kaufman JL, Bahlis NJ, Lonial S, Lee KP. The tao of myeloma. Blood. 2014;124:1873–9.
Orlowski RZ, Stinchcombe TE, Mitchell BS, Shea TC, Baldwin AS, Stahl S, et al. Phase I trial of the proteasome inhibitor PS-341 in patients with refractory hematologic malignancies. J Clin Oncol. 2002;20:4420–7.
Aghajanian C, Soignet S, Dizon DS, Pien CS, Adams J, Elliott PJ, et al. A phase I trial of the novel proteasome inhibitor PS341 in advanced solid tumor malignancies. Clin Cancer Res. 2002;8:2505–11.
Siegel DS, Martin T, Wang M, Vij R, Jakubowiak AJ, Lonial S, et al. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood. 2012;120:2817–25.
Vij R, Wang M, Kaufman JL, Lonial S, Jakubowiak AJ, Stewart AK, et al. An open-label, single-arm, phase 2 (PX-171-004) study of single-agent carfilzomib in bortezomib-naive patients with relapsed and/or refractory multiple myeloma. Blood. 2012;119:5661–70.
Mitsiades CS, Mitsiades NS, McMullan CJ, Poulaki V, Kung AL, Davies FE, et al. Antimyeloma activity of heat shock protein-90 inhibition. Blood. 2006;107:1092–100.
Richardson PG, Badros AZ, Jagannath S, Tarantolo S, Wolf JL, Albitar M, et al. Tanespimycin with bortezomib: activity in relapsed/refractory patients with multiple myeloma. Br J Haematol. 2010;150:428–37.
Vatolin S, Phillips JG, Jha BK, Govindgari S, Hu J, Grabowski D, et al. Novel protein disulfide isomerase inhibitor with anticancer activity in multiple myeloma. Cancer Res. 2016;76:3340–50.
Xu S, Butkevich AN, Yamada R, Zhou Y, Debnath B, Duncan R, et al. Discovery of an orally active small-molecule irreversible inhibitor of protein disulfide isomerase for ovarian cancer treatment. Proc Natl Acad Sci USA. 2012;109:16348–53.
Ge J, Zhang CJ, Li L, Chong LM, Wu X, Hao P, et al. Small molecule probe suitable for in situ profiling and inhibition of protein disulfide isomerase. ACS Chem Biol. 2013;8:2577–85.
Allimuthu D, Adams DJ. 2-Chloropropionamide as a low-reactivity electrophile for irreversible small-molecule probe identification. ACS Chem Biol. 2017;12:2124–31.
Galligan JJ, Petersen DR. The human protein disulfide isomerase gene family. Hum Genom. 2012;6:6.
Hoffstrom BG, Kaplan A, Letso R, Schmid RS, Turmel GJ, Lo DC, et al. Inhibitors of protein disulfide isomerase suppress apoptosis induced by misfolded proteins. Nat Chem Biol. 2010;6:900–6.
Kim HT, Russell RL, Raina AK, Harris PL, Siedlak SL, Zhu X, et al. Protein disulfide isomerase in Alzheimer disease. Antioxid Redox Signal. 2000;2:485–9.
Honjo Y, Ito H, Horibe T, Takahashi R, Kawakami K. Protein disulfide isomerase-immunopositive inclusions in patients with Alzheimer disease. Brain Res. 2010;1349:90–6.
Conn KJ, Gao W, McKee A, Lan MS, Ullman MD, Eisenhauer PB, et al. Identification of the protein disulfide isomerase family member PDIp in experimental Parkinson’s disease and Lewy body pathology. Brain Res. 2004;1022:164–72.
Jasuja R, Passam FH, Kennedy DR, Kim SH, van Hessem L, Lin L, et al. Protein disulfide isomerase inhibitors constitute a new class of antithrombotic agents. J Clin Invest. 2012;122:2104–13.
Cho J, Furie BC, Coughlin SR, Furie B. A critical role for extracellular protein disulfide isomerase during thrombus formation in mice. J Clin Invest. 2008;118:1123–31.
Gallina A, Hanley TM, Mandel R, Trahey M, Broder CC, Viglianti GA, et al. Inhibitors of protein-disulfide isomerase prevent cleavage of disulfide bonds in receptor-bound glycoprotein 120 and prevent HIV-1 entry. J Biol Chem. 2002;277:50579–88.
Barbouche R, Miquelis R, Jones IM, Fenouillet E. Protein-disulfide isomerase-mediated reduction of two disulfide bonds of HIV envelope glycoprotein 120 occurs post-CXCR4 binding and is required for fusion. J Biol Chem. 2003;278:3131–6.
Xu S, Sankar S, Neamati N. Protein disulfide isomerase: a promising target for cancer therapy. Drug Discov Today. 2014;19:222–40.
Kurtoglu M, Philips K, Liu H, Boise LH, Lampidis TJ. High endoplasmic reticulum activity renders multiple myeloma cells hypersensitive to mitochondrial inhibitors. Cancer Chemother Pharmacol. 2010;66:129–40.
Horna-Terron E, Pradilla-Dieste A, Sanchez-de-Diego C, Osada J. TXNDC5, a newly discovered disulfide isomerase with a key role in cell physiology and pathology. Int J Mol Sci. 2014;15:23501–18.
Watanabe MM, Laurindo FR, Fernandes DC. Methods of measuring protein disulfide isomerase activity: a critical overview. Front Chem. 2014;2:73.
Rutkevich LA, Cohen-Doyle MF, Brockmeier U, Williams DB. Functional relationship between protein disulfide isomerase family members during the oxidative folding of human secretory proteins. Mol Biol Cell. 2010;21:3093–105.
Presolski SI, Hong VP, Finn MG. Copper-catalyzed azide-alkyne click chemistry for bioconjugation. Curr Protoc Chem Biol. 2011;3:153–62.
Shevchenko A, Tomas H, Havlis J, Olsen JV, Mann M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat Protoc. 2006;1:2856–60.
Llarrull LI, Toth M, Champion MM, Mobashery S. Activation of BlaR1 protein of methicillin-resistant Staphylococcus aureus, its proteolytic processing, and recovery from induction of resistance. J Biol Chem. 2011;286:38148–58.
Ren G, Champion MM, Huntley JF. Identification of disulfide bond isomerase substrates reveals bacterial virulence factors. Mol Microbiol. 2014;94:926–44.
Holmgren A. Thioredoxin catalyzes the reduction of insulin disulfides by dithiothreitol and dihydrolipoamide. J Biol Chem. 1979;254:9627–32.
Stessman HA, Lulla A, Xia T, Mitra A, Harding T, Mansoor A, et al. High-throughput drug screening identifies compounds and molecular strategies for targeting proteasome inhibitor-resistant multiple myeloma. Leukemia. 2014;28:2263–7.
Thompson RM, Dytfeld D, Reyes L, Robinson RM, Smith B, Manevich Y, et al. Glutaminase inhibitor CB-839 synergizes with carfilzomib in resistant multiple myeloma cells. Oncotarget. 2017;8:35863–76.
Speers AE, Cravatt BF. Profiling enzyme activities in vivo using click chemistry methods. Chem Biol. 2004;11:535–46.
Laurindo FR, Pescatore LA, Fernandes Dde C. Protein disulfide isomerase in redox cell signaling and homeostasis. Free Radic Biol Med. 2012;52:1954–69.
Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284:13291–5.
Kaplan A, Gaschler MM, Dunn DE, Colligan R, Brown LM, Palmer AG, et al. Small molecule-induced oxidation of protein disulfide isomerase is neuroprotective. Proc Natl Acad Sci USA. 2015;112:E2245–52.
Bekendam RH, Bendapudi PK, Lin L, Nag PP, Pu J, Kennedy DR, et al. A substrate-driven allosteric switch that enhances PDI catalytic activity. Nat Commun. 2016;7:12579.
Banerjee R, Pace NJ, Brown DR, Weerapana E. 1,3,5-Triazine as a modular scaffold for covalent inhibitors with streamlined target identification. J Am Chem Soc. 2013;135:2497–500.
Khan MM, Simizu S, Lai NS, Kawatani M, Shimizu T, Osada H. Discovery of a small molecule PDI inhibitor that inhibits reduction of HIV-1 envelope glycoprotein gp120. ACS Chem Biol. 2011;6:245–51.
Chesi M, Robbiani DF, Sebag M, Chng WJ, Affer M, Tiedemann R, et al. AID-dependent activation of a MYC transgene induces multiple myeloma in a conditional mouse model of post-germinal center malignancies. Cancer Cell. 2008;13:167–80.
Chesi M, Matthews GM, Garbitt VM, Palmer SE, Shortt J, Lefebure M, et al. Drug response in a genetically engineered mouse model of multiple myeloma is predictive of clinical efficacy. Blood. 2012;120:376–85.
Baell JB, Holloway GA. New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J Med Chem. 2010;53:2719–40.
Baell J, Walters MA. Chemistry: chemical con artists foil drug discovery. Nature. 2014;513:481–3.
Okumura M, Kadokura H, Inaba K. Structures and functions of protein disulfide isomerase family members involved in proteostasis in the endoplasmic reticulum. Free Radic Biol Med. 2015;83:314–22.
Laboissiere MC, Sturley SL, Raines RT. The essential function of protein-disulfide isomerase is to unscramble non-native disulfide bonds. J Biol Chem. 1995;270:28006–9.
Jurczyszyn A, Zebzda A, Czepiel J, Perucki W, Bazan-Socha S, Cibor D, et al. Geldanamycin and its derivatives inhibit the growth of myeloma cells and reduce the expression of the MET receptor. J Cancer. 2014;5:480–90.
Stuhmer T, Zollinger A, Siegmund D, Chatterjee M, Grella E, Knop S, et al. Signalling profile and antitumour activity of the novel Hsp90 inhibitor NVP-AUY922 in multiple myeloma. Leukemia. 2008;22:1604–12.
Lancet JE, Gojo I, Burton M, Quinn M, Tighe SM, Kersey K, et al. Phase I study of the heat shock protein 90 inhibitor alvespimycin (KOS-1022, 17-DMAG) administered intravenously twice weekly to patients with acute myeloid leukemia. Leukemia. 2010;24:699–705.
Peterson LB, Eskew JD, Vielhauer GA, Blagg BS. The hERG channel is dependent upon the Hsp90alpha isoform for maturation and trafficking. Mol Pharm. 2012;9:1841–6.
Khandelwal A, Kent CN, Balch M, Peng S, Mishra SJ, Deng J, et al. Structure-guided design of an Hsp90beta N-terminal isoform-selective inhibitor. Nat Commun. 2018;9:425.
Que NLS, Crowley VM, Duerfeldt AS, Zhao J, Kent CN, Blagg BSJ. et al. Structure based design of a Grp94-selective inhibitor: exploiting a key residue in Grp94 to optimize paralog-selective binding. J Med Chem. 2018;61:2793–805.
Lin L, Gopal S, Sharda A, Passam F, Bowley SR, Stopa J, et al. Quercetin-3-rutinoside inhibits protein disulfide isomerase by binding to its b’x domain. J Biol Chem. 2015;290:23543–52.
Acknowledgements
N.G.D. is an investigator in the South Carolina Center of Biomedical Research Excellence (COBRE) in Oxidants, Redox Balance and Stress Signaling (P20GM103542) and is a Research Scholar of the American Cancer Society (RSG-14-156-01-CDD). This work was also supported by NIH/NCI (1R41CA213488-01), the South Carolina Clinical & Translational Research Institute with an academic home at the Medical University of South Carolina (MUSC; UL1 RR029882 and UL1 TR000062), shared resources of the MUSC Hollings Cancer Center (P30 CA138313), and by the Hollings Cancer Center T32 Ruth L. Kirschstein National Research Service Award Training Program T32 (CA193201). We would also like to thank Pablo Sobrado at Virginia Tech for the generous gifts of the pVP56K vector and the TEV-containing pVP67K vector.
Author contributions
RMR, LR, RMD, HB, MES, and NGD performed research. ABR directed synthesis of E64FC26. JJM and CJC provided support for organic synthesis of E61 derivatives. YM provided support for ROS assays. MMC performed MS of proteins from pull-downs. MC and PLB directed Vk*Myc experiments. RMR and NGD designed research, analyzed data, and wrote the manuscript. All authors reviewed and authorized the final version.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
RMR and NGD are inventors on patents. NGD has an ownership interest in Leukogene Therapeutics, Inc. The other authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Robinson, R.M., Reyes, L., Duncan, R.M. et al. Inhibitors of the protein disulfide isomerase family for the treatment of multiple myeloma. Leukemia 33, 1011–1022 (2019). https://doi.org/10.1038/s41375-018-0263-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-018-0263-1
This article is cited by
-
Functional characterization of the dimeric form of PDGF-derived fusion peptide fabricated based on theoretical arguments
Scientific Reports (2024)
-
Functions and mechanisms of protein disulfide isomerase family in cancer emergence
Cell & Bioscience (2022)
-
Comparison of anti-cancer effects of novel protein disulphide isomerase (PDI) inhibitors in breast cancer cells characterized by high and low PDIA17 expression
Cancer Cell International (2022)
-
The deubiquitinase OTUD1 regulates immunoglobulin production and proteasome inhibitor sensitivity in multiple myeloma
Nature Communications (2022)
-
PDI inhibitor LTI6426 enhances panobinostat efficacy in preclinical models of multiple myeloma
Cancer Chemotherapy and Pharmacology (2022)