Abstract
Improving outcomes in acute myeloid leukemia (AML) remains a major clinical challenge. Overexpression of pro-survival BCL-2 family members rendering transformed cells resistant to cytotoxic drugs is a common theme in cancer. Targeting BCL-2 with the BH3-mimetic venetoclax is active in AML when combined with low-dose chemotherapy or hypomethylating agents. We now report the pre-clinical anti-leukemic efficacy of a novel BCL-2 inhibitor S55746, which demonstrates synergistic pro-apoptotic activity in combination with the MCL1 inhibitor S63845. Activity of the combination was caspase and BAX/BAK dependent, superior to combination with standard cytotoxic AML drugs and active against a broad spectrum of poor risk genotypes, including primary samples from patients with chemoresistant AML. Co-targeting BCL-2 and MCL1 was more effective against leukemic, compared to normal hematopoietic progenitors, suggesting a therapeutic window of activity. Finally, S55746 combined with S63845 prolonged survival in xenograft models of AML and suppressed patient-derived leukemia but not normal hematopoietic cells in bone marrow of engrafted mice. In conclusion, a dual BH3-mimetic approach is feasible, highly synergistic, and active in diverse models of human AML. This approach has strong clinical potential to rapidly suppress leukemia, with reduced toxicity to normal hematopoietic precursors compared to chemotherapy.
Similar content being viewed by others
Introduction
Acute myeloid leukemia (AML) is a hematopoietic malignancy arising from the transformation of myeloid progenitor cells. Recurrent mutations in over 70 genes characterize the genomic landscape of AML, with each patient harboring a unique spectrum of ancestral, clonal, and sub-clonal populations that evolve over time and in relation to selective pressures exerted by chemotherapy [1, 2]. Although treatment options have remained static for decades, the FDA has recently approved four AML therapies in 2017, including midostaurin, CPX-351, gemtuzumab ozogomycin, and enasidenib [3]. Another promising strategy involves targeting pro-survival activity in AML with BH3-mimetics designed to target BCL-2 and related family members [4]. The most clinically advanced drug is venetoclax, which selectively targets BCL-2 and is approved for clinical use in chronic lymphocytic leukemia [5]. S55746/BCL201 is an orally active, selective, and potent inhibitor of BCL-2 that impairs hematological tumor growth and is currently in phase 1 clinical development [6, 7]. Other BH3-mimetics selectively targeting other pro-survival proteins include A1331852, which inhibits BCL-XL and the recently described MCL1 inhibitor S63845, which our group has shown to be well tolerated in mice and active against a number of malignancies, including a subset of AML at low nanomolar concentrations [8, 9].
Although single agent clinical activity with venetoclax has been modest in AML, clinical responses are increased in combination with either hypomethylating agents or low-dose cytarabine for treatment-naive elderly patients ineligible for intensive chemotherapy [10,11,12]. In addition to BCL-2, MCL1 is frequently co-expressed in AML and has been shown to play a critical pro-survival role in AML [13]. Chemotherapy enhances anti-leukemic activity by upregulating TP53 and increasing expression of downstream BH3-only members NOXA and PUMA, which are capable of targeting pro-survival proteins not neutralized by venetoclax, such as MCL1 [14, 15]. Venetoclax resistance is associated with upregulation of pro-survival BCL-XL or MCL1, supporting the rationale to target multiple pro-survival proteins simultaneously [13, 14, 16, 17]. We have recently shown that combined targeting of BCL-2 with venetoclax and direct inhibition of MCL1 with a lentiviral BH3-expressing vector was highly effective in producing prolonged remissions in xenograft models of AML [14]. Although synergy between BH3-mimetics targeting BCL-2 and MCL1 has been previously reported in AML, prior MCL1 inhibitors have not been suitable for clinical development [18].
The potential to clinically target MCL1 was realized by development of the potent and selective small molecule inhibitor S63845 [8]. Although we previously showed that pharmacological targeting of MCL1 was tolerated by normal human CD34 + precursors, the tolerability of targeting multiple pro-survival proteins using BH3-mimetics has not been reported [18]. We now show that simultaneous inhibition of both MCL1 (with S63845) and BCL-2 (with venetoclax or S55746) is highly synergistic in AML and extends low nanomolar activity with the combination to approximately half the primary AML samples tested, spanning a broad spectrum of cytogenetic and molecular profiles. S55746 in combination with S63845 was also active against chemotherapy relapsed and refractory AML samples, indicating therapeutic potential for patients with chemoresistant disease. Synergy with S63845 was stronger in combination with BCL-2 inhibitors than with cytotoxic or hypomethylating agents. Simultaneous BH3-mimetic targeting of BCL-2 and MCL1 produced rapid and durable remissions in cell line xenograft models and bone marrow cytoreductions in patient-derived xenograft (PDX) models of AML. Despite robust activity against leukemic progenitors, pharmacologic inhibition of BCL-2/MCL1 was less toxic to normal CD34 + progenitors than standard cytotoxic drugs. Our results support the clinical development of a novel, non-chemotherapy based approach, combining BH3-mimetics to target both BCL-2 and MCL1 in patients with AML.
Materials and methods
AML cell lines
MV4;11, MOLM-13, PL-21, ML-2, Nomo-1, THP-1, EOL-1, Kasumi-1 (from ATCC) were cultured in RPMI (GIBCO) supplemented with FBS 10% (v/v); HL-60, KG1, KG1a were cultured in IMDM (GIBCO) supplemented with FBS 20% (v/v); and OCI-AML3 in MEM alpha (GIBCO) supplemented with FBS 20% (v/v). In addition, all media contained penicillin (100 IU/ml), streptomycin (100 µg/ml), and L-glutamine (2 mM). All cell lines were determined to be free of mycoplasma contamination.
Drugs, cell viability, and synergy assays
S55746 and S63845 were provided by Servier Laboratories. A1331852 was synthesized as previously described [9]. Venetoclax was purchased from Active Biochem (Cat. No. A-1231). Decitabine was purchased from Abcam (Cat. No. ab120842), Idarubicin was purchased from Selleckchem (Cat. No. S1228), Cytarabine was purchased from Hospira. Bone marrow samples from patients with AML and donor CD34 + cells were collected after informed consent in accordance with guidelines approved by The Alfred and Royal Melbourne Hospital human research ethics committees. Ficoll-purified and red cell depleted AML cells were plated in RPMI and 15% FBS at 2.5 × 105 cells/mL and drugs tested over a 5-log concentration range. After 48 h, cell viability was determined by FACS analysis of cellular exclusion of SYTOX Blue Dead Cell Stain (Life Technologies Cat No S34857) using an LSR-Fortessa (BD) [19, 20]. FACS data was analyzed using the FlowJo software. GraphPad Prism software was used to calculate drug concentrations causing 50% lethality (LC50).
For the larger scale drug synergy assays, cells were seeded and treated with nine 2-fold serial dilutions of each compound dispensed into cell assay plates. Single agent IC50’s were calculated using standard four-parametric curve fitting. IC50 was defined as the compound concentration at which the Cell Titer Glo (CTG) signal was reduced to 50% of that measured for the vehicle (DMSO) control. In order to analyze the activity of the compounds in combination, the cells were seeded and treated with seven or eight 3.16-fold serial dilutions of each compound dispensed, either individually or in all possible permutations in a checkerboard fashion. Effects of the single agents, as well as their checkerboard combinations on cell viability were assessed after three days of incubation at 37 °C/5% CO2 using CTG at 75 μL reagent/well. Two independent experiments, each one performed in duplicate, were performed. Luminescence was quantified on a multipurpose plate reader. Potential synergistic interactions between compound combinations were assessed using the Loewe additivity model (ChaliceTM Bioinformatics Software available in Horizon website) and reported as the Synergy Score [20]. Cell viability assays for LC50 determination and colony forming unit (CFU) assays have been previously described [18].
Targeted sequencing
Genomic DNA libraries were prepared using either a custom Haloplex (Agilent) panel, as previously described [21], or the TruSight Myeloid Sequencing Panel (Illumina) according to the manufacturer’s instructions. Sequencing was carried out on the Illumina NextSeq 500 platform with 150-bp paired-end reads. The resulting FASTQ files were aligned using the TruSeq Amplicon App (version 3.0.0) on BaseSpace (Illumina) and variants called using the Somatic Variant Caller (Illumina). The vcf files generated were filtered in Variant Studio (Illumina) and further verified using the Integrative Genomics Viewer (Broad Institute).
Mouse AML xenograft models
All studies in mice were performed under the institutional guidelines approved by the Alfred Medical Research and Education Precinct Animal Ethics Committee. MV4;11 cells transduced with the luciferase reporter (pLUC2 promega) were intravenously injected at 1 × 105 cells into 6-week old female and male irradiated (100 Rad) non-obese diabetic/severe combined immunodeficient (NOD/SCID/IL2rγnull) mice as previously described [21]. Engraftment was measured on day 7 (MV4;11) or day 38 (OCI-AML3) by quantifying the percentage of hCD45 + cells in the peripheral blood (PB) by flow cytometry and by IVIS imaging of bioluminescent MV4;11 or OCI-AML3 cells. After detectable engraftment, mice were gavaged with S55746 (200 µL 100 mg/kg) dissolved in PEG400 (Sigma), absolute ethanol (Sigma), and distilled H20 at a ratio of 40:10:50 or S63845 (200 µL 25 mg/kg) twice weekly or weekly IV dissolved in 50% 2-hydroxypropyl)-β-cyclodextrin (Sigma) and 50% 50 mM HCL or the drug combination, or vehicle. Blood counts were determined using a hematology analyzer (BioRad, Gladesville, NSW). Bioluminescent imaging was performed using the Caliper IVIS Lumina III XR imaging system. Mice were anesthetized with isoflurane, injected intraperitoneally with 100 µL of 125 mg/kg luciferin (Perkin Elmer, Springvale, VIC), and BLI performed 5 minutes later.
Patient-derived xenograft models of AML
To establish mouse models of primary patient AML, 1 × 106 leukemic blasts were injected into 6-week old female NOD-IL2Rcγnull (NRG-SG3) mice (The Jackson Laboratory, Bar Harbor, ME, USA) via tail-vein injection and animals monitored for leukemia progression using flow cytometric analysis of peripheral blood for hCD45 + cells. hCD45 + cell counts in the bone marrow from the femurs of euthanized animals were used to determine the extent of leukemia infiltration. Bone marrow cells were extracted by flushing femurs in PBS supplemented with 2% fetal bovine serum. To determine the efficacy of S55746 and S63845, mice were gavaged daily with S55746 (100 mg/kg) for 5 days or received S63845 25 mg/kg twice weekly IV. Drug efficacy was determined by flow cytometric analysis of hCD45 + cells in bone marrow isolated from flushed femurs. Sternums and spleens were fixed in formalin, sectioned, and stained with hematoxylin and eosin or anti-hCD45 to assess leukemic burden and cellularity.
For additional methods refer to Supplementary Information.
Results
Combined targeting of BCL-2 and MCL1 induces potent and synergistic apoptosis in primary AML samples
We first compared the pro-survival dependency of primary AML samples treated with a panel of potent and selective inhibitors of MCL1 (S63845), BCL-2 (ABT-199/venetoclax or S55746/BCL201), or BCL-XL (A1331852). Comparison was made with idarubicin and cytarabine, drugs commonly used in the treatment of AML. Freshly derived primary AML samples were studied if the viability of the cells in control media plus DMSO after 48 h was at least 70%. Primary samples were derived from patients with treatment naive (Fig. 1a) or chemotherapy relapsed/refractory (Fig. 1b) AML.
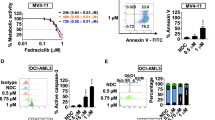
Interrogating pro-survival dependency in AML using BH3-mimetic drugs alone and in combination. The sensitivity (LC50) of freshly derived primary samples to BH3-mimetics alone, or in equimolar combination, relative to chemotherapy (cytarabine and idarubicin) after 48 h exposure. Samples are separated according to whether patients were a chemotherapy naive or had b relapsed and refractory AML. The control cell viability of each AML sample after 48 h in DMSO is shown. The upper concentration of cytarabine tested was 100 µM and for other drugs 10 µM. A color bar grading the LC50 values for each drug in the heat map is shown. c MV4;11 and OCI-AML3 cells were treated with indicated drugs and the LC50 at 16 h determined. Where indicated, cells were pre-incubated with the caspase inhibitor QVD (50 μM) for 1 h prior to addition of the other drugs. d Synergistic interactions between S63845 combined with standard anti-leukemic drugs were assessed using an Excess Inhibition matrix according to the Loewe additivity model. Synergy scores (SS) are represented as the mean of n = 2; each experiment being performed in duplicate (SS = 0 represents an additive effect, SS > 2 represents synergy; hashed line and SS > 5 represents strong synergy; dotted line)
Consistent with previously published work, a minor proportion of primary AML cases appeared highly sensitive (LC50 < 100 nM) to BCL-2 (venetoclax or S55746), or MCL1 (S63845) targeting alone [8]. Fewer primary samples were sensitive to the BCL-XL inhibitor A1331852 (Fig. 1a, b). The activities of the BCL-2 inhibitors venetoclax and S55746 appeared highly correlated (Fig. 1a, b and Supp. Figure 1A). In contrast to modest single agent activity observed from targeting BCL-2, BCL-XL, or MCL1 alone, marked anti-leukemic activity (LC50 sensitivity < 100 nM) was apparent in > 50% of the primary AML samples when treated simultaneously with equimolar concentrations of BH3-mimetics targeting BCL-2 and MCL1 (Fig. 1a, b). Similar activity was observed for S63845 combined with either venetoclax or the BCL-2 inhibitor S55746. In contrast, combined inhibition of pro-survival BCL-XL and MCL1 was less effective, suggesting that in the majority of AML cases, pro-survival function in AML was predominantly mediated by BCL-2 and MCL1. Co-targeting BCL-2 and MCL1 was also efficacious (LC50 < 100 nM) in a subset of primary AML samples resistant to the anthracycline drug idarubicin (LC50 > 1 μM; Fig. 1a, b). The sensitivity of AML samples to combined S55746 and S63845 was similar among samples from patients treatment naïve or relapsed/refractory to prior chemotherapy (Fig. 1a, b and Supp. Figure 1B). Leukemic cell death from combined S55746 and S63845 therapy was caspase (Fig. 1c and Supp. Figure 1C, D) and BAX/BAK dependent (Supp. Figure 1E, F). In summary, our findings suggest that BCL-2 and MCL1 are key determinants of pro-survival activity in both treatment naive and chemoresistant AML and that simultaneous targeting of both proteins appears necessary for optimal pro-apoptotic activity [14].
Anti-leukemic synergy of S63845 combined with BCL-2 inhibition compared to standard drugs
Comparative drug synergy experiments between the MCL1 inhibitor S63845 and standard drugs was not possible using primary AML samples, due to insufficient cell numbers. Therefore, a series of 12 AML cell lines was employed to assess the activity of S63845 in combination with S55746, cytotoxic drugs (idarubicin, cytarabine), or the hypomethylating agent decitabine (Fig. 1d). Synergistic interactions were assessed using the Loewe additivity model to derive Synergy Scores (SS); with a SS > 2 indicating synergy and a SS > 5 strong synergy [22]. Single agent LC50 and representative effect and synergy matrices for the cell line OCI-AML3 treated with S63845 and S55746 are shown (Supp. Figure 2A,B). The strongest synergistic interaction was observed between S63845 and S55746 (SS > 5 in 12/12 AML cell lines tested). In comparison, for S63845 in combination with decitabine, synergy scores > 5 were observed in 4 out of 12 AML cell lines tested, whereas for S63845 combined with cytotoxic drugs idarubicin or cytarabine, most synergy scores were < 5 (Fig. 1d).
Combined targeting of BCL-2 and MCL1 is efficacious in poor risk AML
Poor survival outcomes in AML are associated with adverse-risk chromosomal lesions (complex karyotype, aneuploidy, t(6;9), inv(3), MLL-fusions), mutations involving TP53 or the RNA splicing/chromatin machinery (RUNX1, ASXL1, BCOR, STAG2, EZH2, SRSF2, SF3B1, U2AF1, ZRSR2 or MLLPTD) [1, 23]. Therefore, the cytoreductive effect of dual BCL-2 and MCL1 targeting was explored across treatment naïve and chemotherapy relapsed/refractory AML samples characterized for cytogenetic abnormalities and recurrent mutations using a 54-gene targeted exome panel (Fig. 2) [24]. Sensitivity to combined BCL-2/MCL1 targeting (LC50 < 100 nM) was observed across a broad spectrum of AML cases, including those with recognized poor risk genomic features, such as mutated RUNX1 (11/15 cases sensitive), DNMT3A (10/16 cases sensitive) or ASXL1 (7/14 cases sensitive)(Fig. 2) [1]. A notable observation was potential resistance to combined BCL-2/MCL1 targeting (LC50 > 100 nM) among cases harboring mutant TP53 (7/8 resistant). As overall numbers were limited, an expanded sample set will be required to confirm this preliminary association.
Mutation profiling of primary AML samples and sensitivity to BCL-2/MCL1 targeting. Sensitivity to combined S55746/S63845 is shown, with sensitive samples (LC50 < 100 nM) colored red and resistant cases (LC50 > 100 nM) green. Prior patient resistance to chemotherapy is indicated by orange colored boxes. Mutation presence is indicated by filled black boxes for each sample. Cytogenetic abnormalities are summarized, with gray shading indicating adverse-risk karyotype. Missing FLT3-ITD values are indicated (NA)
Leukemic stem and progenitor cells were more sensitive to BCL-2/MCL1 targeting than normal CD34+progenitors
We have previously shown that a small subset of primary AML samples are sensitive to the MCL1 inhibitor S63845 in long-term clonogenic assays, with less toxicity observed in normal donor CD34 + cells, highlighting a therapeutic window for MCL1 targeting leukemic progenitors [8]. In contrast, cytotoxic drugs cytarabine, and idarubicin were toxic to both leukemic and normal progenitors, consistent with the known severe myelosuppressive effects associated with these drugs [8]. We now show that combined targeting of BCL-2 and MCL1 dramatically enhances activity against AML progenitors, compared to either drug given alone (Fig. 3a, b, d). The combination suppressed clonogenic activity (by > 50%) in 5/7 primary AML cases tested, with minimal impact on the clonogenic growth of normal CD34 + cells (Fig. 3d). In contrast, BCL-2 or MCL1 targeting alone only suppressed clonogenic activity in 2–3/8 cases each. Interestingly, the selective BCL-XL inhibitor A1331852 was toxic to both leukemic and normal progenitors in clonogenic assays (Fig. 3c). These findings demonstrate that combined BCL-2/MCL1 targeting has the best therapeutic index for suppressing human leukemic stem and progenitor cells.
Comparison of the effects of BCL-2 and MCL-1 targeting on leukemic compared to normal progenitor function. Suppression of clonogenic activity by a S55746 b S63845 c A1331852, or d combined S55746/S63845. Between 104–105 primary AML or normal donor CD34 + cells (3–4 donors) were plated in 0.6% agar supplemented with GM-CSF, IL-3, SCF, and EPO and cultured for 2–3 weeks at 37 °C with 100 nM of each BH3-mimetic. Colonies were enumerated by GelCountTM and normalized to the number of colonies from primary AML or CD34 + cells treated with DMSO. AML colony numbers (as a % normalized to DMSO) are shown with error bars indicating mean + /− 1 s.d. of each AML sample performed in duplicate. In the CD34 + column for each drug, colony numbers (as a % normalized to DMSO) from duplicate experiments from 2 or 3 donors are pooled and shown. To facilitate comparison, Fig. 3b includes data from our prior publication [8] with the addition of two new samples
Combined therapy with S55746 and S63845 has potent anti-leukemic activity in vivo
Our previous work showed that mice engrafted with MV4;11 and OCI-AML3 cells could achieve sustained remissions if BCL-2 and MCL1 were concomitantly targeted [14]. This previous study employed venetoclax to target BCL-2 in combination with a lentiviral BIM-BH3 vector designed to conditionally target MCL1 in vivo [14]. The limitation of this experiment was that MCL1 was continuously targeted by the lentivirus, which does not reflect how a drug delivered intermittently would work. We therefore sought to determine whether pharmacological targeting of BCL-2 and MCL1 using a small molecule approach could similarly enhance survival in animal model systems of AML. Preliminary in vitro studies confirmed the efficacy of combined BCL-2 and MCL1 targeting with S55746 and S63845, respectively, to enhance caspase–dependent pro-apoptotic activity of both MV4;11 and OCI-AML3 cell lines (Fig. 1c). To demonstrate synergistic activity in vivo, immunodeficient NSG mice were engrafted with luciferase-labeled MV4;11 or OCI-AML3 cells and monitored by bioluminescence imaging (BLI) for tumor development in vivo. Whole body imaging identified foci of engrafted bioluminescent-avid MV4;11 tumors by day 7 and OCI-AML3 tumors by day 32 post-transplant, respectively (Fig. 4a, b). Mouse cohorts with established leukemia were then treated with either S55746, S63845, or the combination of both drugs. In mice xenografted with MV4;11 AML, eradication of bioluminescent-avid disease was most effective with combined S55746 and S63845 (Fig. 4a). Although partial early suppression of MV4;11 leukemia was achieved by S55746 or S63845 monotherapy, this did not translate into significant improvements in long-term survival (Fig. 4c). Combined S55746/S63845, however, increased median survival of established AML over three-fold in the MV4;11 model, even though treatment ceased on day 35 (Fig. 4c).
Combined targeting of BCL-2 and MCL1 improves the survival of mice xenografted with human AML (a) 24 irradiated NSG mice were transplanted with 105 human MV4;11 cells transduced with a luciferase reporter construct. AML engraftment was confirmed by bioluminescence imaging on day 7. On day 10 (arrow), mice were divided into treatment groups of six mice and treated with (i) vehicle, (ii) S55746 100 mg/kg by oral gavage daily (5 days/week for 4 weeks), (iii) S63845 25 mg/kg IV twice weekly for 4 weeks, or (iv) combination S55746/S63845 for 4 weeks, with treatment ending on day 35. b Similar experiment as in (A) using OCI-AML3 cells transduced with a luciferase reporter. Engraftment was confirmed on day 32. Treatment commenced on day 38 (arrow) post-transplant for a total of 7 weeks. c NSG mice engrafted with human MV4;11 and treated as in (a) and followed for Kaplan–Meier (KM) survival (ethical endpoints) showing that combined treatment with S55746/S63845 resulted in significantly longer survival than vehicle control (arrows show start and end of treatment). d KM survival of NSG mice engrafted with human OCI-AML3 cells and treated as in (a) showing that combined treatment with S55746/S63845 resulted in significantly longer survival than vehicle control (arrows show start and end of treatment)
In the OCI-AML3 model, combined S55746/S63845 was also more effective at suppressing AML than either drug alone as assessed by BLI (Fig. 4b). In contrast to the MV4;11 model, residual BLI-avidity for OCI-AML3 tumor was still evident after 4 weeks of treatment with S55746/S63845 (Fig. 4b). OCI-AML3 cells are more resistant in vitro and express higher levels of MCL1 than MV4;11 cells (Figure Suppl. Figure 2A and not shown). Therefore, treatment commenced on day 38 and was continued for 7 weeks. This resulted in 50% survival by day 125 in mice treated with combined S55746 and S63845, compared to no surviving mice beyond day 75 in vehicle and single agent arms (Fig. 4d).
Combination therapy with S55746/S63845 suppresses patient-derived AML in vivo
To examine the potential for dual BCL-2 and MCL1 targeted therapy to suppress patient-derived AML in vivo, primary AML samples were xenografted into immunodeficient NOD.Rag1−/−;γcnull (NRG)-SG3 mice, which have been transgenically modified to express human SCF, GM-CSF, and IL3 in vivo, to enhance engraftment of human AML blasts [25, 26]. Strikingly, in two independent PDX models of AML (AML 01-254-2014 and AML01-173-2015), S55746/S63845 led to cytoreduction of AML after only 5 days of treatment as assessed by immunohistological staining of mouse sternums (Fig. 5a, b) or flow cytometric enumeration of human CD45 + blasts from flushed femurs (Fig. 5c, d). In AML 01-254-2014, the anti-leukemic effect of dual BCL-2/MCL1 targeting appeared more effective than a 5-day schedule of decitabine and comparable to the effect of decitabine in combination with BH3-mimetics targeting either BCL-2 or MCL1 (Fig. 5d).
Combined S55746 and S63845 suppresses leukemia in vivo in NRG-SG3 patient-derived xenograft models of AML. a Irradiated NRG-SG3 mice were transplanted with 106 primary AML cells (AML01-173-2015). Engraftment was confirmed at 6 weeks by detection of hCD45 in peripheral blood. Cohorts of 2–4 mice per group were then treated with (i) vehicle (d1–5), (ii) S55746 100 mg/kg days 1–5 by gavage, (iii) S63845 25 mg/kg IV on days 2 and 4), or (iv) S55746 combined with S63845. Mice were euthanized on day 8 and immunohistological analysis for hCD45 + performed on sternal bone marrow for infiltration by AML cells captured at ×100 magnification using the Aperio ScanScope. Two representative cases from each cohort are shown. b Similar experiment as in (a) with NRG-SG3 mice engrafted with AML 01-254-2014 and treated with (i) vehicle, (ii) S55746, (iii) S63845, or (iv) S55746 combined with S63845. Flow cytometric analysis of flushed femurs on day 8 showing the percentage of human CD45 + blasts from AML 01-173-2015 (c) after (i) vehicle, (ii) S55746, (iii) S63845, and (iv) S55746 combined with S63845. Similar experiment in d using AML 01-254-2014 showing the effects of (i) vehicle, (ii) S55746 (iii) S63845 (iv) S55746 combined with S63845, (v) decitabine 0.4 mg/kg IV days 1–5, (vi) decitabine plus S55746, or (vii) decitabine plus S63845. In (c, d) mean, + /- 1 s.d. from individual mouse values are shown
Combined BCL-2 and MCL1 targeting was less toxic to engrafted normal human hematopoiesis than chemotherapy in vivo
Having demonstrated the potential for dual BH3-mimetic targeting of BCL-2 and MCL1 to rapidly induce remissions and extend the survival of mice xenografted with AML, we next examined the tolerance of normal hematopoietic cells to combined S55746/S63845 therapy. Prior published conditional Mcl1 gene knockout studies suggested that long-term Mcl1 ablation was poorly tolerated by mouse hematopoietic cells [27]. The MCL1 inhibitor S63845 targets human MCL1 with 6-fold higher affinity than mouse Mcl1 [8]. We therefore sought to verify the effect of pharmacologic targeting of both MCL1 and BCL-2 on human hematopoietic function by engrafting NSG mice with CD34 + cells from a normal human donor. After confirmation of engraftment, mice were treated with S55746 and S63845 over a five-day period. In contrast to marked suppression of AML in vivo (Fig. 5), immunohistological staining of engrafted donor CD34 + cells in sternal bone marrow (Fig. 6a and Suppl Fig. 3) and flow cytometric enumeration of flushed femurs (Fig. 6b) showed minimal impact of combined S55746/S63845 treatment on normal CD45 + cells. In contrast, decitabine therapy was toxic (Fig. 6a, b). Collectively, these results suggest that targeting both BCL2 and MCL1 using small molecule inhibitors to suppress human AML in vivo may have less severe adverse effects on normal bone marrow hematopoietic function than standard cytotoxic drugs.
Combined S63845 and S55746 did not affect normal hematopoietic cell function. a Irradiated NSG mice were transplanted with 105 normal donor CD34 + progenitor cells. Following detectable engraftment in peripheral blood at week 20, mice were treated with (i) S55746 100 mg/kg/d by oral gavage (days 1–5), (ii) S63845 25 mg/kg IV (days 1 and 4), (iii) combination S55746/S63845, or (iv) decitabine 0.4 mg/kg intraperitoneally (days 1–5). Mice were euthanized on day 8 and immunohistological hCD45 + analysis performed on sternal bone marrow and spleen sections captured at ×100 magnification using the Aperio ScanScope. Due to limited availability of donor CD34+cells, 2 mice were engrafted and treated in arms i–iii and one mouse in arm iv; representative images are shown with additional examples in Suppl Fig. 3. b Representative flow cytometric dot plots of flushed femurs from mice treated as indicated in (a) are shown displaying the proportion of marrow cells expressing human versus mouse CD45
Discussion
Since its inception in the 1970’s, combined cytarabine/anthracycline or “7 + 3” chemotherapy has been an enduring constant in the therapy of AML. Our current work highlights several new findings of translational importance for patients with AML. We demonstrate for the first time that small molecule BH3-mimetics directly targeting BCL-2 and MCL1 can potently suppress human AML, with limited toxicity to normal human hematopoietic progenitors, potentially overcoming one of the major limitations from the use of cytotoxic drugs. Furthermore, primary AML blasts were more sensitive to combined MCL1/BCL-2 than to combined MCL1/BCL-XL targeting. This avoids undesirable on-target thrombocytopenia that may result from BCL-XL inhibition [28]. Our studies also demonstrate the anti-leukemic activity of a new BCL-2 inhibitor (S55746), in combination with the recently described MCL1 inhibitor S63845. The translational potential of these findings in AML are high, as both S55746 (NCT02920541) and the clinical MCL1 inhibitor derivative S64315 (NCT02979366) are both currently undergoing phase 1 clinical evaluation in human AML.
Although venetoclax has previously been shown to synergistically enhance the activity of the MCL1 inhibitor A-1210477, the latter was only been shown to be effective in vitro and against AML cell lines at micromolar concentrations [29]. In contrast, the current work shows that combined S55746 and S63845 has low nanomolar activity across a broad spectrum of AML genotypes, including primary samples from patients with adverse genetic risk and chemoresistant AML. A proportion of primary AML samples remained resistant to BCL-2/MCL1 targeting (Fig. 2). Our gene mutation profiling studies showed enrichment of samples with TP53 mutation in this sub-group. The mechanistic basis of this resistance remains to be determined. Possible causes include expression of non-targeted pro-survival factors, such as BFL-1 or BCL-XL, the requirement to target multiple pro-survival proteins simultaneously (e.g., BCL-2/BCL-XL/MCL1) or defective expression of downstream BAX or BAK. Examination of these possibilities will be the subject of future research.
Prior to this study, the feasibility of targeting both BCL-2 and MCL1, even with pharmacological inhibitors, remained uncertain. Previous lineage-specific deletion models indicated potential risk to cardiac [30, 31], granulocyte/hematopoietic [27, 32,33,34], lymphocyte/thymocyte [35, 36], neuronal [37], and liver function [38, 39] as a consequence of long-term MCL1 ablation. We have recently shown that an intermittent weekly or twice weekly schedule of a potent short-acting pharmacological inhibitor of MCL1 is well tolerated in animal models and active against a range of cancers in vivo, including AML [8]. The short protein half-life of MCL1 permits rapid regeneration in critical organs, potentially supporting physiological tolerance to short-term S63845 exposure [40]. Until now, pulsatile inhibition of BCL-2 and MCL1 mimicking a drug-like effect has not been possible using genetically engineered approaches. The availability of potent and selective small molecule inhibitors of BCL-2, BCL-XL, and MCL1 enables determination of which pro-survival proteins are important for the survival of normal, as well as malignant tissues, both in vitro and in vivo. Importantly, the sensitivity of intact cells to BH3-mimetics can be measured quantitatively, utilizing compounds intended for clinical use.
In conclusion, our studies using S55746 and S63845 provide proof-of-concept demonstration that targeting BCL-2 and MCL1 simultaneously can lead to rapid suppression of diverse AML subtypes, with limited toxicity to normal human bone marrow cells, thereby providing strong rationale for further clinical development.
References
Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic classification and prognosis in acute myeloid leukemia. New Engl J Med. 2016;374:2209–21.
Ding L, Ley TJ, Larson DE, et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature. 2012;481:506–10.
Wei AH, Tiong S. Midostaurin, enasidenib, CPX-351, gemtuzumab ozogomycin and venetoclax bring new hope to AML. Blood. 2017;130:2469–74. blood-2017-2008-784066
Vo T-T, Ryan J, Carrasco R, et al. Relative mitochondrial priming of myeloblasts and normal HSCs determines chemotherapeutic success in AML. Cell . 2012;151:344–55.
Roberts AW, Davids MS, Pagel JM, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. New Engl J Med. 2015;374:311–22.
Wei A, Bajel A, Boissel N, et al. Preliminary results from a phase 1 study examining the novel BCL-2 inhibitor S55746/BCL201 as single agent in patients with acute myeloid leukemia or high risk myelodysplastic syndrome. Haematologica. 2017;102:373–373.
Casara P, Davidson J, Claperon A, et al. S55746 is a novel orally active BCL-2 selective and potent inhibitor that impairs hematological tumor growth. Oncotarget. 2018;9:20075–88.
Kotschy A, Szlavik Z, Murray J, et al. The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature. 2016;538:477–82.
Leverson JD, Phillips DC, Mitten MJ, et al. Exploiting selective BCL-2 family inhibitors to dissect cell survival dependencies and define improved strategies for cancer therapy. Sci Transl Med. 2015;7:279ra240.
Wei A, Strickland SA, Roboz GJ, et al. Safety and efficacy of venetoclax plus low-dose cytarabine in treatment-naive patients aged ≥65 years with acute myeloid leukemia. Blood. 2016;128:102–102.
Pollyea D, DiNardo C, Thirman MJ, et al. A phase 1b study of venetoclax (ABT-199/GDC-0199) in combination with decitabine or azacitidine in treatment-naive patients with acute myelogenous leukemia who are ≥to 65 years and not eligible for standard induction therapy. J Clin Oncol. 2016;34((suppl):abstr 7009.
Konopleva M, Pollyea DA, Potluri J, et al. Efficacy and biological correlates of response in a phase II study of venetoclax monotherapy in patients with acute myelogenous leukemia. Cancer Discov. 2016;6:1106–17.
Glaser SP, Lee EF, Trounson E, et al. Anti-apoptotic Mcl-1 is essential for the development and sustained growth of acute myeloid leukemia. Genes & Dev. 2012;26:120–5.
Teh T-C, Nguyen NY, Moujalled DM, Segal D, Pomilio G, Rijal S, et al. Enhancing venetoclax activity in acute myeloid leukemia by co-targeting MCL1. Leukemia . 2018;32:303–12.
Niu X, Zhao J, Ma J, et al. Binding of released Bim to Mcl-1 is responsible for resistance to ABT-199 which can be overcome by combination with daunorubicin or cytarabine in acute myeloid leukemia cells. Blood. 2015;126:1265–1265.
Zhang Q, Pan R, Han L, et al. Mechanisms of acquired resistance to venetoclax in preclinical AML models. Blood. 2015;126:328–328.
Lin KH, Winter PS, Xie A, et al. Targeting MCL-1/BCL-XL forestalls the acquisition of resistance to ABT-199 in acute myeloid leukemia. Sci Rep. 2016;6:27696.
Leverson J, Zhang H, Chen J, et al. Potent and selective small-molecule MCL-1 inhibitors demonstrate on-target cancer cell killing activity as single agents and in combination with ABT-263 (navitoclax). Cell Death & Dis. 2015;6:e1590.
Thomas D, Powell JA, Vergez F, et al. Targeting acute myeloid leukemia by dual inhibition of PI3K signaling and Cdk9-mediated Mcl-1 transcription. Blood. 2013;122:738–48.
Powell JA, Thomas D, Barry EF, et al. Expression profiling of a hemopoietic cell survival transcriptome implicates osteopontin as a functional prognostic factor in AML. Blood. 2009;114:4859–70.
Jin L, Lee EM, Ramshaw HS, et al. Monoclonal antibody-mediated targeting of CD123, IL-3 receptor α chain, eliminates human acute myeloid leukemic stem cells. Cell Stem Cell. 2009;5:31–42.
Lehár J, Krueger AS, Avery W, et al. Synergistic drug combinations tend to improve therapeutically relevant selectivity. Nat Biotechnol. 2009;27:659–66.
Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–47.
Ivey A, Hills RK, Simpson MA, et al. Assessment of minimal residual disease in standard-risk AML. New Engl J Med. 2016;374:422–33.
Wunderlich M, Chou F-S, Link K, et al. AML xenograft efficiency is significantly improved in NOD/SCID-IL2RG mice constitutively expressing human SCF, GM-CSF and IL-3. Leukemia. 2010;24:1785–9.
Wunderlich M, Brooks RA, Panchal R, Rhyasen GW, Danet-Desnoyers G, Mulloy JC. OKT3 prevents xenogeneic GVHD and allows reliable xenograft initiation from unfractionated human hematopoietic tissues. Blood. 2014;123:e134–e144.
Opferman J, Iwasaki H, Ong C, et al. Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. Science’s Stke. 2005;307:1101.
Mason KD, Carpinelli MR, Fletcher JI, et al. Programmed anuclear cell death delimits platelet life span. Cell . 2007;128:1173–86.
Luedtke DA, Niu X, Pan Y, et al. Inhibition of Mcl-1 enhances cell death induced by the Bcl-2-selective inhibitor ABT-199 in acute myeloid leukemia cells. Signal Transduct Target Ther. 2017;2:17012.
Wang X, Bathina M, Lynch J, et al. Deletion of MCL-1 causes lethal cardiac failure and mitochondrial dysfunction. Genes & Dev. 2013;27:1351–64.
Thomas RL, Roberts DJ, Kubli DA, et al. Loss of MCL-1 leads to impaired autophagy and rapid development of heart failure. Genes & Dev. 2013;27:1365–77.
Dzhagalov I, John AS, He Y-W. The antiapoptotic protein Mcl-1 is essential for the survival of neutrophils but not macrophages. Blood. 2007;109:1620–6.
Steimer DA, Boyd K, Takeuchi O, Fisher JK, Zambetti GP, Opferman JT. Selective roles for antiapoptotic MCL-1 during granulocyte development and macrophage effector function. Blood. 2009;113:2805–15.
Opferman JT, Iwasaki H, Ong CC, et al. Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. Science. 2005;307:1101–4.
Dunkle A, Dzhagalov I, He Y-W. Mcl-1 promotes survival of thymocytes by inhibition of Bak in a pathway separate from Bcl-2. Cell Death & Differ. 2010;17:994–1002.
Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426:671.
Arbour N, Vanderluit JL, Le Grand JN, et al. Mcl-1 is a key regulator of apoptosis during CNS development and after DNA damage. J Neurosci. 2008;28:6068–78.
Hikita H, Takehara T, Shimizu S, et al. Mcl‐1 and Bcl‐xL cooperatively maintain integrity of hepatocytes in developing and adult murine liver. Hepatology. 2009;50:1217–26.
Vick B, Weber A, Urbanik T, et al. Knockout of myeloid cell leukemia‐1 induces liver damage and increases apoptosis susceptibility of murine hepatocytes. Hepatology. 2009;49:627–36.
Yang T, Buchan HL, Townsend KJ, Craig RW. MCL‐1, a member of the BCL‐2 family, is induced rapidly in response to signals for cell differentiation or death, but not to signals for cell proliferation. J Cell Physiol. 1996;166:523–36.
Acknowledgements
We acknowledge research funding from National Health and Medical Research Council, Victorian Cancer Agency, Leukaemia Foundation of Australia, Leukemia and Lymphoma Society, Australian Cancer Research Foundation, Monash Partners, the Alfred Foundation and the Medical Research Future Fund. This work has received funding support from Servier.
Author contributions
AW and OG conceived and designed the research; DMM, GP, CG, AI, JS, SR, SM, FR, and AZ performed the research; LZ, I-ST, PL, DS, CG, and ALM provided reagents and database information, AW, DMM, DH, AWR, ALM, GL, OG, MC, AC, LK-B, YW, EH, EM, and FC analyzed and interpreted the data; AW, DMM, AWR, DH, AM, OG, MC, AC, LK-B, YW, EH, EM, and FC wrote the paper; and all authors approved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
AW is a principal investigator in clinical trials involving S55746 and venetoclax in AML. DH, GL, and AWR are employees of the Walter and Eliza Hall Institute, which receives milestone and royalty payments related to venetoclax, and that AW, AWR, and DH have received research funding from Servier. CG, MC, AC, FR, AZ, LK-B, FC, AM, and OG are employees of Servier. YW, EH, and EM are employees of Novartis.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moujalled, D.M., Pomilio, G., Ghiurau, C. et al. Combining BH3-mimetics to target both BCL-2 and MCL1 has potent activity in pre-clinical models of acute myeloid leukemia. Leukemia 33, 905–917 (2019). https://doi.org/10.1038/s41375-018-0261-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-018-0261-3
This article is cited by
-
Inhibition of NRF2 enhances the acute myeloid leukemia cell death induced by venetoclax via the ferroptosis pathway
Cell Death Discovery (2024)
-
BCL-2 protein family: attractive targets for cancer therapy
Apoptosis (2023)
-
Identification of bicyclic compounds that act as dual inhibitors of Bcl-2 and Mcl-1
Molecular Diversity (2023)
-
Dual targeting of BCL-2 and MCL-1 in the presence of BAX breaks venetoclax resistance in human small cell lung cancer
British Journal of Cancer (2023)
-
Potent, p53-independent induction of NOXA sensitizes MLL-rearranged B-cell acute lymphoblastic leukemia cells to venetoclax
Oncogene (2022)