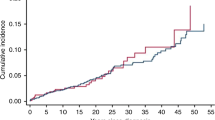
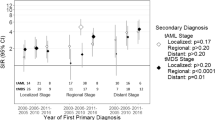
Abstract
Although increased risk of acute myeloid leukemia (AML) has been observed after chemotherapy and radiotherapy, less is known about radiotherapy-related risks of specific AML subtypes and other specific myeloid neoplasms. We used the US population-based cancer registry data to evaluate risk of myeloid neoplasms among three cohorts of cancer survivors initially treated with radiotherapy only. We included 1-year survivors of first primary thyroid (radioiodine only, stages I–IV; N = 49 879), prostate (excluding stage IV; N = 237 439), or uterine corpus cancers (stage I–II; N = 16 208) diagnosed during 2000–2013. We calculated standardized incidence ratios (SIRs) and excess absolute risks (EARs). Thyroid cancer survivors had significantly elevated risks of total AML (SIR = 2.77, 95% CI: 1.99–3.76), AML with cytogenetic abnormalities (SIR = 3.90, 95% CI: 1.57–8.04), AML with myelodysplasia-related changes (SIR = 2.87, 95% CI: 1.05–6.25), and BCR-ABL1-positive chronic myelogenous leukemia (CML) (SIR = 5.38, 95% CI: 2.58–9.89). Irradiated prostate and uterine corpus cancer survivors were at elevated risk for total AML (SIR = 1.14, 95% CI: 1.03–1.27 and SIR = 1.77, 95% CI: 1.01–2.87, respectively), AML with cytogenetic abnormalities (SIR = 2.52, 95% CI: 1.84–3.37 and SIR = 7.21, 95% CI: 2.34–16.83, respectively), and acute promyelocytic leukemia (SIR = 3.20, 95% CI: 2.20–4.49 and SIR = 8.88, 95% CI: 2.42–22.73, respectively). In addition, prostate cancer survivors were at increased risk of BCR-ABL1-positive CML (SIR = 2.11, 95% CI: 1.52–2.85). Our findings support the importance of diagnostic precision in myeloid neoplasm classification since susceptibility following radiotherapy may vary by myeloid neoplasm subtype, thereby informing risk/benefit discussions in first primary cancer treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Bryant AK, Banegas MP, Martinez ME, Mell LK, Murphy JD. Trends in radiation therapy among cancer survivors in the United States, 2000-2030. Cancer Epidemiol Biomarkers Prev. 2017;26:963–70.
Berrington de Gonzalez A, Gilbert E, Curtis R, Inskip P, Kleinerman R, Morton L, et al. Second solid cancers after radiation therapy: a systematic review of the epidemiologic studies of the radiation dose-response relationship. Int J Radiat Oncol Biol Phys. 2013;86:224–33.
Leone G, Fianchi L, Pagano L, Voso MT. Incidence and susceptibility to therapy-related myeloid neoplasms. Chem Biol Interact. 2010;184:39–45.
Morton LM, Dores GM, Tucker MA, Kim CJ, Onel K, Gilbert ES, et al. Evolving risk of therapy-related acute myeloid leukemia following cancer chemotherapy among adults in the United States, 1975-2008. Blood. 2013;121:2996–3004.
Hsu WL, Preston DL, Soda M, Sugiyama H, Funamoto S, Kodama K, et al. The incidence of leukemia, lymphoma and multiple myeloma among atomic bomb survivors: 1950-2001. Radiat Res. 2013;179:361–82.
Iwanaga M, Hsu WL, Soda M, Takasaki Y, Tawara M, Joh T, et al. Risk of myelodysplastic syndromes in people exposed to ionizing radiation: a retrospective cohort study of Nagasaki atomic bomb survivors. J Clin Oncol. 2011;29:428–34.
Curtis RE, Boice JD Jr., Stovall M, Bernstein L, Holowaty E, Karjalainen S, et al. Relationship of leukemia risk to radiation dose following cancer of the uterine corpus. J Natl Cancer Inst. 1994;86:1315–24.
Howard R, Gilbert E, Lynch CF, Hall P, Storm H, Holowaty E, et al. Risk of leukemia among survivors of testicular cancer: a population-based study of 42,722 patients. Ann Epidemiol. 2008;18:416–21.
Lonn S, Gilbert ES, Ron E, Smith SA, Stovall M, Curtis RE. Comparison of second cancer risks from brachytherapy and external beam therapy after uterine corpus cancer. Cancer Epidemiol Biomarkers Prev. 2010;19:464–74.
Radivoyevitch T, Sachs RK, Gale RP, Molenaar RJ, Brenner DJ, Hill BT, et al. Defining AML and MDS second cancer risk dynamics after diagnoses of first cancers treated or not with radiation. Leukemia. 2016;30:285–94.
Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov). SEER*Stat Database: Incidence - SEER 18 Regs excluding AK, Nov 2016 Sub (1973-2014 varying) Katrina/Rita Population Adjustment: Linked To County Attributes—Total U.S., 1969-2015 Counties. National Cancer Institute, DCCPS, Surveillance Research Program, released April 2017, based on the November 2016 submission.
Fritz A, Percy C, Jack, A, Shanmugaratnam K, Sobin L, Parkin DM, Whelan S, editors. International classification of diseases for oncology. 3rd edn. Geneva: World Health Organization; 2000.
Swerdlow S, Campo E, Harris N, Jaffe E, Pileri S, Stein H, et al., editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: International Agency for Research on Cancer; 2008.
Jaffe E, Harris NL, Stein H, Vardiman JW, editors. WHO classification of tumours. Pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyon: International Agency for Research on Cancer; 2001.
Fraumeni Jr JF, Curtis RE, Edwards BK, Tucker MA. Introduction. In: Curtis RE, Freedman DM, Ron E, Ries LAG, Hacker DG, Edwards BK, et al., editors. New malignancies among cancer survivors: SEER cancer registries, 1973-2000. Bethesda: National Cancer Institute; 2006 (NIH Publ No. 05-5302).
Yasui Y, Liu Y, Neglia JP, Friedman DL, Bhatia S, Meadows AT, et al. A methodological issue in the analysis of second-primary cancer incidence in long-term survivors of childhood cancers. Am J Epidemiol. 2003;158:1108–13.
Ronckers CM, McCarron P, Ron E. Thyroid cancer and multiple primary tumors in the SEER cancer registries. Int J Cancer. 2005;117:281–8.
Sawka AM, Thabane L, Parlea L, Ibrahim-Zada I, Tsang RW, Brierley JD, et al. Second primary malignancy risk after radioactive iodine treatment for thyroid cancer: a systematic review and meta-analysis. Thyroid. 2009;19:451–7.
Seo GH, Cho YY, Chung JH, Kim SW. Increased risk of leukemia after radioactive iodine therapy in patients with thyroid cancer: a nationwide, population-based study in Korea. Thyroid. 2015;25:927–34.
Teng CJ, Hu YW, Chen SC, Yeh CM, Chiang HL, Chen TJ, et al. Use of radioactive iodine for thyroid cancer and risk of second primary malignancy: a nationwide population-based study. J Natl Cancer Inst. 2016;108:djv314.
Molenaar RJ, Pleyer C, Radivoyevitch T, Sidana S, Godley A, Advani AS, et al. Risk of developing chronic myeloid neoplasms in well-differentiated thyroid cancer patients treated with radioactive iodine. Leukemia. 2017 ;32:952–9.
Molenaar RJ, Sidana S, Radivoyevitch T, Advani AS, Gerds AT, Carraway HE, et al. Risk of hematologic malignancies after radioiodine treatment of well-differentiated thyroid cancer. J Clin Oncol. 2017:JCO2017750232. https://doi.org/10.1200/JCO.2017.75.0232. [Epub ahead of print].
Hall P, Boice JD Jr, Berg G, Bjelkengren G, Ericsson UB, Hallquist A, et al. Leukaemia incidence after iodine-131 exposure. Lancet. 1992;340:1–4.
Iyer NG, Morris LG, Tuttle RM, Shaha AR, Ganly I. Rising incidence of second cancers in patients with low-risk (T1N0) thyroid cancer who receive radioactive iodine therapy. Cancer. 2011;117:4439–46.
Mukherjee S, Reddy CA, Ciezki JP, Abdel-Wahab M, Tiu RV, Copelan E, et al. Risk for developing myelodysplastic syndromes in prostate cancer patients definitively treated with radiation. J Natl Cancer Inst. 2014;106:djt462.
Ojha RP, Fischbach LA, Zhou Y, Felini MJ, Singh KP, Thertulien R. Acute myeloid leukemia incidence following radiation therapy for localized or locally advanced prostate adenocarcinoma. Cancer Epidemiol. 2010;34:274–8.
Wang R, Zeidan AM, Yu JB, Soulos PR, Davidoff AJ, Gore SD, et al. Myelodysplastic syndromes and acute myeloid leukemia after radiotherapy for prostate cancer: a population-based study. Prostate. 2017;77:437–45.
Berrington de Gonzalez A, Wong J, Kleinerman R, Kim C, Morton L, Bekelman JE. Risk of second cancers according to radiation therapy technique and modality in prostate cancer survivors. Int J Radiat Oncol Biol Phys. 2015;91:295–302.
Park JH, Qiao B, Panageas KS, Schymura MJ, Jurcic JG, Rosenblat TL, et al. Early death rate in acute promyelocytic leukemia remains high despite all-trans retinoic acid. Blood. 2011;118:1248–54.
Pearce MS, Salotti JA, Little MP, McHugh K, Lee C, Kim KP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012;380:499–505.
Dores GM, Devesa SS, Curtis RE, Linet MS, Morton LM. Acute leukemia incidence and patient survival among children and adults in the United States, 2001-2007. Blood. 2012;119:34–43.
Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood. 2006;107:265–76.
Srour SA, Devesa SS, Morton LM, Check DP, Curtis RE, Linet MS, et al. Incidence and patient survival of myeloproliferative neoplasms and myelodysplastic/myeloproliferative neoplasms in the United States, 2001-12. Br J Haematol. 2016;174:382–96.
Curtis RE, Ries LAG. Methods. In: Curtis RE, Freedman DM, Ron E, Ries LAG, Hacker DG, Edwards BK, et al., editors. New malignancies among cancer survivors: SEER cancer registries, 1973-2000. Bethesda; National Cancer Institute; 2006 (NIH Publ No. 05-5302).
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–405.
Nardi V, Winkfield KM, Ok CY, Niemierko A, Kluk MJ, Attar EC, et al. Acute myeloid leukemia and myelodysplastic syndromes after radiation therapy are similar to de novo disease and differ from other therapy-related myeloid neoplasms. J Clin Oncol. 2012;30:2340–7.
Duffield AS, Aoki J, Levis M, Cowan K, Gocke CD, Burns KH, et al. Clinical and pathologic features of secondary acute promyelocytic leukemia. Am J Clin Pathol. 2012;137:395–402.
Espirito Santo A, Chacim S, Ferreira I, Leite L, Moreira C, Pereira D, et al. Effect of therapy-related acute myeloid leukemia on the outcome of patients with acute myeloid leukemia. Oncol Lett. 2016;12:262–8.
Granfeldt Ostgard LS, Medeiros BC, Sengelov H, Norgaard M, Andersen MK, Dufva IH, et al. Epidemiology and clinical significance of secondary and therapy-related acute myeloid leukemia: a national population-based cohort study. J Clin Oncol. 2015;33:3641–9.
Zeidan AM, Al Ali N, Barnard J, Padron E, Lancet JE, Sekeres MA, et al. Comparison of clinical outcomes and prognostic utility of risk stratification tools in patients with therapy-related vs de novo myelodysplastic syndromes: a report on behalf of the MDS Clinical Research Consortium. Leukemia. 2017;31:1391–7.
Acknowledgements
This study was supported by the Intramural Program of the National Cancer Institute, National Institutes of Health, Department of Health and Human Services and by the Dutch Cancer Society (Grant No. DCOG2011-5027 and UVA2012-5517).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Teepen, J.C., Curtis, R.E., Dores, G.M. et al. Risk of subsequent myeloid neoplasms after radiotherapy treatment for a solid cancer among adults in the United States, 2000–2014. Leukemia 32, 2580–2589 (2018). https://doi.org/10.1038/s41375-018-0149-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-018-0149-2