Abstract
Progestin resistance is the main obstacle for the conservative therapy to maintain fertility in women with endometrial cancer. Brusatol was identified as an inhibitor of the NRF2 pathway; however, its impact on progestin resistance and the underlying mechanism remains unclear. Here, we found that brusatol sensitized endometrial cancer to progestin by suppressing NRF2-TET1-AKR1C1-mediated progestin metabolism. Brusatol transcriptionally suppressed AKR1C1 via modifying the hydroxymethylation status in its promoter region through TET1 inhibition. Suppression of AKR1C1 by brusatol resulted in decreased progesterone catabolism and maintained potent progesterone to inhibit endometrial cancer growth. This inhibition pattern has also been found in the established xenograft mouse and organoid models. Aberrant overexpression of AKR1C1 was found in paired endometrial hyperplasia and cancer samples from the same individuals with progestin resistance, whereas attenuated or loss of AKR1C1 was observed in post-treatment samples with well progestin response as compared with paired pre-treatment tissues. Our findings suggest that AKR1C1 expression pattern may serve as an important biomarker of progestin resistance in endometrial cancer.
Similar content being viewed by others
Introduction
Epidemiologic studies have revealed that atypical hyperplasia and well-differentiated cancer tend to occur in younger women1,2. These women, especially those of child-bearing age who wish to become pregnant, have a strong desire to maintain their fertility. For these individuals, conservative management with progestin is the optimal choice. However, ~30% of the patients develop progestin resistance3.
In the past decades, several mechanisms have been proposed to explain progestin resistance, such as deficiency of progestin receptor (PR), downregulation of ERα expression, aberrant survivin expression as well as increased TGF-EGFR signaling3,4,5,6,7,8. Recently, downregulation of NRF2 was stated to potentially improve the response of patients with endometrial cancer to progestin therapy or chemotherapy, while high levels of NRF2 contribute to therapy resistance9,10, which may represent another molecular mechanism involved in progestin resistance.
AKR1C1 is well characterized as a target gene of NRF211,12. Increased AKR1C1 expression has been described in a previous study and might be associated with the pathological progression of endometrial cancer13,14. AKR1C1 functions as 20α-hydroxysteroid dehydrogenase and inactivates progesterone by forming 20α-dihydroxyprogesterone, a metabolite with weak progestin function15,16. In this reaction, AKR1C1 exhibits a high catalytic efficiency with a KM of 5.7 µM and a kcat 0.93/min17. Although the mechanism has been illustrated in diseased endometrium that overexpression of AKR1C1 contributes to diminished protective effect of progesterone by inactivating progesterone13, whether AKR1C1 is associated with progestin resistance in endometrial cancer is not clear.
We have stated that TET1 silence impairs NRF2 expression and subsequently found that TET1-mediated hydroxymethylaiton involves in regulation of NRF2 transcriptional activity18. TET1 is an important components of the ten-eleven translocation 5-methylcytosine dioxygenase family, which catalyzes the conversion of 5-mC to 5-hmC19. Aberrant expression of TET1 is associated with the development of multiple types of cancer20,21. In our previous study, TET1 has been found to play a role in metformin-enhanced progestin sensitivity4. We have also demonstrated that TET1-dependent DNA hydroxymethylation contributes to elevated NRF2 expression in endometrial cancer18. Therefore, the role of TET1 in NRF2-driven progestin resistance needs to be further clarified.
Brusatol was first discovered by Ren et al. and identified as an inhibitor of the NRF2 pathway. It sensitizes multiple types of cancer cells to anti-cancer drugs by downregulating NRF2 via ubiquitination-dependent degradation22. Many studies showed that brusatol can potently decrease the expression of other proteins including HIF-1α, p38, STAT3 and SQSTM123,24,25, which implies that brusatol is a global protein synthesis inhibitor26,27,28. Despite ubiquitination-dependent degradation is an important manner for brusatol to suppress protein expression, increasing evidences illustrated brusatol can also regulate its targets at the transcriptional level. However, little is currently known about how brusatol transcriptionally mediates its targets and whether brusatol is involved in NRF2-driven progestin resistance.
In our reports, AKR1C1 was identified as a key scavenger of progestin and a mediator of NRF2-TET1 driven progestin resistance. Aberrant expression of AKR1C1 was observed in endometrial cancer patient samples with poor progestin response, which suggests that AKR1C1 is a specific marker to identify progestin resistance. Moreover, progestin resistance due to the NRF2-TET1-AKR1C1 signaling axis can be reversed by brusatol in precancerous and cancerous endometrial cells.
Materials and methods
Cell lines and cell culture
Human endometrial cancer cell lines (Ishikawa and ECC1) were maintained in our laboratory. HEK-293 cells were purchased from American Type Culture Collection (Manassas, VA). The cells were cultured in DMEM: F12 medium (1:1, GIBCO) containing 10% fetal bovine serum (Gibco, Gaithersburg, MD, USA), 100 U/ml penicillin G and 100 µg/ml streptomycin (Life Technologies, Inc., Rockville, MD) and placed in a 37 °C incubator with a humidified atmosphere containing 5% CO2.
Establishment of stable cell lines, transient transfection, small interfering RNA transfection and progestin treatment
Stable cell lines with NRF2/AKR1C1 overexpression or AKR1C1 depletion were established using a retrovirus system as described previously9,10. Transient transfection of the indicated plasmids or siRNA was performed with LipofectamineTM 3000 (Invitrogen, Carlsbad, CA, USA).
Drug treatment and cell proliferation assay
Endometrial cancer cells were treated with MPA, brusatol, or tBHQ for the indicated times. Cell proliferation was measured with a CCK8 assay.
Immunoblot analysis
Cells were lysed with RIPA buffer to extract total protein. In total, 50 µg of protein per lane was loaded onto an SDS-polyacrylamide gel, electrophoresed and transferred to PVDF membranes, which were incubated overnight with primary antibodies. After the membranes were washed and treated with the indicated secondary antibodies, detection of the protein bands was carried out using a chemiluminescence detection system (ECL detection kit; Pierce, Rockford, IL). Each experiment was performed at least three times.
Dot blot and hMeDIP assay
We extracted total DNA and performed a gradient dilution of the samples. The dilutions of total DNA were dropped on nitrocellulose membranes, which were baked at 80 °C for 10 min. The membrane was blocked with 5% skim milk for 1 h before it was incubated with 5hmC primary antibody (1:500 dilution, Active Motif) overnight at 4 °C. After HRP-conjugated secondary antibody incubation, the membrane was subjected to ECL and scanned to visualized bound antibodies. Methylene blue staining served as a loading control. Quantification of the dot blots from three independent assays was calculated with Gel-Pro analyzer software (Media Cybernetics). The gray intensity of dots sampled with 100 ng DNA was analyzed by one-way analysis of variance. For hMeDIP assay, total DNA was extracted and sheared via ultrasonication. The DNA fragments were incubated with 5hmC antibody (Active Motif) and pulled down to amplify the AKR1C1 gene promoter via real-time PCR. The primers used are listed in Table 1.
ARE constructs and dual luciferase reporter assay
The wild type or mutant TET1 AREs were amplified and cloned into pGL4.27 plasmids (Promega) as previously described18. Ishikawa cells were cotransfected with pGL4.27-TET1-ARE plasmids, pRL-SV40-Renilla plasmid (Promega) and NRF2 plasmid, and the relative luciferase activity was determined by a Dual Luciferase Assay Kit (Promega).
20α-dihydroxyprogesterone concentration detection
Endometrial cancer cells treated with indicated drugs were harvested and lysed to determine the 20α-dihydroxyprogesterone concentration with the analysis kits (XQ-EN15767, Xinquan, Company, Shanghai, China). The detailed procedure has been finished as the manual described. And high performance liquid chromatography (HPLC) also was used to quantified the 20α-dihydroxyprogesterone after treatment the endometrial cancer cell with progesterone alone or combine with brusatol and progesterone. In brief, the specific steps are to add 80% methanol to the standard 20α-dihydroxyprogesterone stock buffer and prepare solutions with final gradient concentrations of 100, 50, 20, 10, 5 and 2 pg/ml, respectively. Then set the instrument parameters, according to the set chromatographic and mass spectrometry conditions, add the prepared standard solution or samples into the injection bottle, and the peak at Rt (retention time) = 2.72 (min) was identified to be 20α hydroxyprogesterone. For data analysis, we use MultiQuant software for integration, get the standard curve, and use the standard curve for content calculation. Through calculation, the equation y = 277.45x − 30.741 (R2 = 0.9996) is obtained. Calculate the 20α dihydroxyprogesterone concentration based on the area under the curve in the figure according to the formula.
Selection of matched cases, tissue processing and immunohistochemical (IHC) analysis
Thirty-four pairs of endometrial samples before and after progestin treatment were assessed in this study. Sixteen patients showed complete response (CR) to progesterone therapy, eight patients showed partial response (PR), and ten patients showed no response (NR).The pathological diagnosis of endometrial hyperplasia was reviewed and confirmed by gynecological pathologists (YJ and WZ) on the basis of the WHO classification. Specifically, the different pathological statuses based on progestin response were defined as follows: NR or residual disease, any architectural abnormalities such as clusters of crowded glands, papillary structures, and complex types of glands with or without cytologic atypia either alone or in combination; PR, no residual hyperplasia but an incomplete response or abnormal glands or any residual architectural abnormalities that do not reach the level of residual disease or nonresponsive disease; and CR, attenuated endometrial glands with decidualized stroma. The IHC assay was performed as previously described3,9,18. The “Index in epithelial/stromal cell” is defined as the H-score of AKR1C1 reactivity graded on a score ranging from 0 to 300 based on the product of staining intensity (0–3) and percentage (0–100) of the cells stained. Score of 0 represented negative expression. A staining intensity score of 1–3 represented weak, moderate and strong staining, respectively. All IHC slides were reviewed independently by two investigators.
Human endometrial organoid culture
According to the previous research29, human endometrial organoid expansion medium (ExM) was as follows: DMEM/F12 glutamine medium (Gibco, USA) with B27 supplement minus vitamin A (Thermo, USA), N2 supplement (Thermo, USA), Primocin 100 μg/ml (Invivogen, France), N-Acetyl-L-cysteine 1.25 mM (Sigma, USA), A83-01 500 nM (MCE, USA), nicotinamide 10 nM (Sigma, USA), rh-EGF 50 ng/ml, rh-Noggin 100 ng/ml, rh-HGF 50 ng/ml, rh-Rspondin-1 500 ng/ml and rh-FGF-10 100 ng/ml (Peprotech, USA). Briefly, fresh endometrial cancer tissues were collected, followed by digested with Collagenase IV (Gibco, USA) and centrifugated. The cell pellets were resuspended in ice-cold Matrigel (Corning, USA) and ExM mix. In total, 50 μl drop of Matrigel–cell suspension were plated into the center of wells of 24-well plate. The plate was placed in the incubator for 20 min for solidification and overlaid with organoid ExM. The medium was changed every 2–3 days. Cultures were passaged by manual pipetting every 7–10 days. Endometrial organoids were replanted on 24-well plate and treated with DMSO, MPA 20 μmol/l, Brusatol 10 nmol/l and both for 3 days. Then the samples were photographed under microscope and collected and fixed with 4% paraformaldehyde on ice, followed by resuspending, centrifugating and embedding the organoids with 3% low melting point agarose. The agarose cube with organoids was dehydrated and paraffin embedded for further HE and IHC staining.
In vivo xenograft mouse model
Nude mice were subcutaneously injected with 1 × 106 endometrial cancer cells. After the mice were treated with MPA and brusatol either alone or in combination for 30 days, they were sacrificed. The tumors were harvested for IHC analysis as previously described22.
Statistical analysis
SPSS 19.0 was used for data analysis. Comparisons of proliferation, the dot blot assay, the dual luciferase reporter assay and the western blot results after various treatments between two groups were made with Student’s t test. p < 0.05 was considered a significant difference when compared with the control group.
Results
Brusatol sensitizes endometrial cancer cell to progestin in vitro
We first determined the effect of brusatol on cell proliferation in endometrial cancer cells. As shown in Fig. 1A, brusatol suppressed cellular growth in a dose-dependent manner. In addition, brusatol significantly suppressed the expression of NRF2 and the relative genes in a dose and time-dependent manner (Fig. 1A lower panel, B). NRF2 is reported to be implicated in chemoresistance. We therefore tried to detect whether brusatol, an NRF2 inhibitor, could enhance progestin sensitivity. Compared with brusatol or MPA alone, brusatol combined with MPA markedly suppressed the growth of endometrial cancer cells (Fig. 1C). Next, Ishikawa-NRF2 cells were established by overexpression of NRF2 and we found cells overexpressing NRF2 were resistant to progestin (MPA) therapy. Importantly, brusatol sensitized Ishikawa-NRF2 cells to MPA (Fig. 1D). The corresponding NRF2 signaling proteins and relative TET1 has been determined by western blot in Ishikawa and Ishikawa-NRF2 cells. MPA suppressed NRF2 expression in Ishikawa cells whereas showed little effect on Ishikawa-NRF2 cells. However, brusatol combined with MPA almost eliminated NRF2 expression in both Ishikawa and Ishikawa-NRF2 cells. Similar changes have also been observed in the protein levels of AKR1C1 and TET1 (Fig. 1E).
A Ishikawa and ECC1 cells were treated with the indicated doses of brusatol for 48 h, and cell viability was estimated by the CCK8 assay (upper panel). The expression of NRF2 related protein was detected by immunoblotting (lower panel). *p < 0.05 compared with the blank control. B Ishikawa and ECC1 cells were treated with 20 nM brusatol for the indicated time and the expression levels of NRF2 and its downstream proteins were analyzed by immunoblotting. C Ishikawa cells were treated with the MPA (20 μM) or brusatol (20 nM) alone, or combined MPA and brusatol for the indicated time, and the CCK8 assay was performed. *p < 0.05. D A stable cell line with high levels of NRF2 was established, and its response to progestin was evaluated by a CCK8 assay. *p < 0.05. E The expression patterns of NRF2, TET1 and AKR1C1 were detected by immunoblotting.
Brusatol impairs NRF2-AKR1C1-mediated progestin metabolism
To assess the mechanism which brusatol reversed progestin resistance, the association between NRF2 and AKR1C1 has been defined firstly. As shown in Supplementary Fig. 1, several AREs have been found in AKR1C1 promoter region, which implies that it is a NRF2 target gene as previously reported11,12. Moreover, knockdown of AKR1C1 by selective small interfering RNAs notably facilitated the suppression on cellular growth in the presence of MPA with or without NRF2 transfection (Fig. 2A), which suggested AKR1C1-mediated NRF2-driven progestin resistance. Next, we tried to figure out whether progestin metabolism alteration by AKR1C1 plays an essential role in the progestin treatment failure. We detected the conversion of progestin to 20α-dihydroxyprogesterone (20α-OHP). Overexpression of AKR1C1 resulted in an increase of 20α-dihydroxyprogesterone and reduced levels of progesterone in cell lysates in a dose-dependent manner (Fig. 2B). Moreover, the HPLC analysis indicated brusatol impaired the reversion of progesterone to 20α-OHP. We found that the level of 20α-dihydroxyprogesterone significantly decreased after adding brusatol (Fig. 2C). Consistent with this result, the increased 20α-OHP and reduced progestin by overexpression of AKR1C1 were effectively blocked by brusatol treatment (Fig. 2D). Thus, we speculate that AKR1C1-mediated progestin metabolism may contribute to sensitivity to progestin therapy as we observed above.
A AKR1C1 was silenced in control and NRF2 overexpressed Ishikawa cells, and a CCK8 assay was performed in the presence of MPA (20 μM). B Overexpression of AKR1C1 increased 20α-dihydroxyprogesterone levels and reduced progesterone levels. *p < 0.05 compared with the blank control. C HPLC analysis indicated brusatol blocked progesterone catabolism. The conversion of progesterone to 20α-dihydroxyprogesterone after brusatol treatment was quantified by calculating the area under the curve (right panel). *p < 0.05. D Ishikawa cells were transfected with AKR1C1 plasmid before they were treated with brusatol in the presence or absence of progesterone. The 20α-dihydroxyprogesterone (left) and progesterone levels (right) were detected. *p < 0.05.
Hydroxymethylation suppression of AKR1C1 promoter region through declined TET1 contributes to brusatol-induced progestin sensitivity
We next tried to explore how brusatol regulated AKR1C1. Our data indicated brusatol reduced the level of AKR1C1 in endometrial cancer cells. Despite ubiquitination-dependent degradation is an important manner for brusatol to suppress the target protein expression, increasing evidences illustrate brusatol can also regulate its targets at the transcriptional level. Methylcytosine dioxygenase TET1 is involved in the epigenetic gene regulation. It catalyzes the conversion of 5-mC to 5-hmC, leading to hydroxymethylation and methylation changes in the promoter region. In our previous study, similar expression profiles between NRF2 and TET1 have been detected by IHC in consecutive sections of endometrial tissue samples from hyperplasia, to EAH, progressed to endometrial carcinoma18. Considering brusatol is an NRF2 inhibitor, we thereby further evaluated the possible regulation relationship between NRF2 and TET1. NRF2 was transfected into Ishikawa and ECC1 cells, and the expression pattern of TET1 and other NRF2 targeting genes was estimated. As shown in Fig. 3A, NRF2 overexpression resulted in a significant increase in the expression of TET1 protein as well as other target proteins, including NQO1, HO1, AKR1C1, and AKR1B10, whereas knocking down NRF2 led to decreased expression of these proteins (Fig. 3A, right panel). Similarly, we transfected endometrial cancer cells with a plasmid overexpressing Keap1, an E3 ligase responsible for the degradation of NRF2, and we then found it reduced the expression of NRF2 and its downstream target molecules, including TET1 (Fig. 3B). Meanwhile, it was found that TET1 also bears four AREs in the promoter region within 5000 bp from the transcription start site (Fig. S2). To identify which ARE is necessary for NRF2 to regulate TET1, four truncations targeting the indicated AREs were constructed (Fig. 3C). The dual luciferase reporter assay revealed that each truncation could respond to NRF2 as long as it contains the ARE1 (Fig. 3D, left). Subsequently, it was found that NRF2 transfection could enhance the luciferase activity in wild type ARE1 reporter plasmid, whereas had little effect on mutant ARE1 (Fig. 3D, right), which implies that ARE1 plays an essential role in NRF2-driven TET1 overexpression.
A Transient ectopic expression of NRF2 elevated the levels of TET1 and other target proteins while depletion of NRF2 by siRNAs suppressed its targets. B Keap1 overexpression suppressed NRF2 signaling and TET1. C Schematic diagrams illustrating the construction of TET1-ARE luciferase reporter plasmids with the indicated AREs. D Luciferase activity in endometrial cancer cells transfected with different TET1-ARE constructs after NRF2 overexpression was assessed by a dual luciferase reporter assay. *p < 0.05 compared with the vector control (left). The effect of NRF2 on wild or mutant TET1 ARE’s luciferase activity(right). *p < 0.05, transfected NQO1 ARE served as a positive control. E Brusatol mitigated the effect of NRF2 by decreasing TET1 and AKR1C1 expression. F Ishikawa cells were transfected with TET1 overexpression plasmid. Cells were then treated with or without 20 nM brusatol and the AKR1C1 level was detected by immunoblot. G Ishikawa cells were treated with the indicated dose of brusatol for 48 h, and 5hmC levels in total DNA were detected by a dot blot assay, *p < 0.05 compared with the blank control. H Brusatol abrogated hydroxymethylation in AKR1C1 promoter region. Cells underwent the indicated treatments were subjected to the hMeDIP assay. *p < 0.05. I Keap1 overexpression or TET1 knockdown inhibited the growth of Ishikawa-NRF2 cells, *p < 0.05 compared with the Vector or siCon group.
We have demonstrated brusatol inhibited TET1 expression (Fig. 1B, E) and we speculated brusatol might regulate TET1 at the transcriptional level. We found elevated NRF2 enhanced TET1 and AKR1C1 expression, and this upregulation can be blocked by brusatol in a dose-dependent manner (Fig. 3E). We subsequently found that overexpression of TET1 induced AKR1C1 expression, while the upregulation was inhibited by brusatol treatment (Fig. 3F). In addition, a dot blot assay indicated brusatol potently decreased the level of total DNA hydroxymethylation in Ishikawa cells (Fig. 3G). We next measured the enrichment of 5-hmC in the genetic regions of AKR1C1 using hMeDIP. Results showed TET1 enhanced hydroxymethylation in AKR1C1 promoter region, while it can be impaired by brusatol (Fig. 3H). To investigate the effect of these regulatory mechanisms on NRF2-mediated progestin resistance and brusatol-induced progestin sensitivity, a cell counting kit-8 (CCK8) assay was performed when Keap1 was overexpressed or TET1 was silenced in Ishikawa-NRF2 cells. Interestingly, either Keap1 overexpression or TET1 knockdown could sensitize endometrial cancer cells to MPA (Fig. 3I). These data suggest that NRF2-TET1-AKR1C1 signal pathway plays a critical role in progestin resistance and brusatol transcriptionally regulates AKR1C1 to reverse the resistance.
Brusatol sensitizes endometrial cancer to progestin ex vivo and in vivo
Our above results have shown that brusatol suppressed progestin metabolism through the AKR1C1 inhibition and sensitized endometrial cancer to progestin. We therefore investigated the effects of brusatol on tumor growth in vivo when the mice were subcutaneously injected with Ishikawa and Ishikawa-NRF2 cells. As shown in Fig. 4A, B, tumor growth in nude mice received Ishikawa cell injection was significantly suppressed when it exposed to combined brusatol and MPA, while treatment with MPA or brusatol alone only slightly inhibited tumor growth. While in mice injected with Ishikawa-NRF2 cells, MPA alone showed little effect on tumor growth; however, brusatol in combination with MPA markedly suppressed tumor growth (Fig. 4B). We further used a human endometrial organoid model to re-evaluate this suppression effect. As shown in Fig. 4C, combined treatment with brusatol and MPA dramatically reduced the number of organoids derived from a type I endometrial cancer patient. H&E staining analyses showed that partial of endometrial cancer glands were reversed as normal glands by combined treatment with brusatol and MPA (Fig. 4D). Meanwhile, a declined expression of AKR1C1 was also observed in the brusatol plus MPA group in the organoid model (Fig. 4E). In summary, our data suggested that brusatol can sensitize endometrial cancer to progestin via downregulating AKR1C1.
A Representative pictures of the xenograft tumor subjected to the indicated treatments. B The final volume of the xenograft tumors was estimated at the time of sacrifice. *p < 0.05. C Numbers of organoids after indicated treatments were shown. *p < 0.05, ***p < 0.001 when compared with the control. These organoids were collected for H&E staining (D) and IHC assay against AKR1C1 (E). Bru represents brusatol. *p < 0.05, ***p < 0.001 when compared with the control.
Validation of AKR1C1 as a prognostic marker of progestin resistance
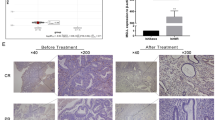
We next tried to validate the significance of AKR1C1 in predicting progestin resistance. It is difficult to determine the local metabolism of progestin in human endometrial lesion tissues, but monitoring the progestin metabolism activity indirectly by detecting AKR1C1 expression profile with IHC assay is reasonable. Thirty-four pairs of endometrial tissues collected before and after progestin treatment were evaluated, and 16 patients showed CR, 8 patients showed PR, and 10 patients showed NR. As shown in Fig. 5A, atypical complex hyperplasia endometrial tissues showed strong AKR1C1 staining, whereas atrophic glands that successfully responded to progestin therapy showed loss of AKR1C1 expression. In cases with PR, we observed normal glands with negative AKR1C1 staining and around hyperplasia glands with strong AKR1C1 expression prior to progestin treatment. After progestin treatment, atrophic glands with negative AKR1C1 staining and the remaining hyperplasia glands with strong staining were observed on the same slide. Moreover, positive AKR1C1 staining was exhibited in tissues pre- and post-progestin treatment from the same patient with poor progestin response, which suggests that AKR1C1 is a potential marker for identifying progestin resistance. The same staining pattern was observed in a case with a PR in which the patient underwent two therapy cycles with progestin; however, the atrophic glands with negative AKR1C1 staining was clearly observed after the second progestin therapy cycle with CR to progestin (Fig. 5B). Interestingly, inverse expression profiles of AKR1C1 in stromal cells and gland cells have been observed, especially in stromal cells with decidual changes around the atrophic endometrial glands, AKR1C1 was positively expressed (Fig. 5C). These data imply that stromal cells play a role in gland epithelial cell proliferation. The expression patterns of AKR1C1 among patients with different responses to progestin are summarized in Table 2.
A Aberrant expression of AKR1C1 in endometrial hyperplasia tissues before and after progestin treatment. IHC assay was used to determine AKR1C1 expression in the patients with different responses to progestin as follows: CR complete response, PR partial response, NR no response. Original magnification in this panel is ×200. B Representative samples from a patient who underwent two progestin treatments cycles and the corresponding PR and CR results were observed. AKR1C1 expression was examined by IHC. Original magnification in the upper panel: ×100, lower panel: ×200. C Inverse expression patterns of AKR1C1 in stromal cells and glands. Red triangle, atrophic gland after progestin treatment with a good response; Blue triangle, hyperplasia gland after progestin treatment with poor response; Purple triangle, normal gland.
Discussion
Women of reproductive age with atypical hyperplasia, endometrial intraepithelial neoplasia, or well-differentiated endometrial cancer have a strong desire to preserve their fertility. Currently, the optimal therapeutic strategy for these women is conservative treatment with a high dose of progestin. However, a low response or resistance to progestin is the main obstacle for successful treatment. Here, we found that brusatol reversed NRF2-driven progestin resistance and suppressed progestin catabolism via AKR1C1 with an epigenetic mechanism (Fig. 6).
NRF2 binds to the AREs in the TET1 promoter region and enhances TET1 expression, which in turn facilitating AKR1C1 expression via hydroxymethylation modification. Furthermore, high levels of NRF2 enhance progestin metabolism by upregulating AKR1C1, which contributes to progestin resistance due to loss of drug function in progestin treatment. This kind of progestin resistance can be reversed by brusatol.
Since progestin resistance abrogates the therapy effect of progestin, numerous studies have focused on how it happens. Reduced PR expression is thought to be one of the critical mechanisms of progestin resistance due to desensitization to progestin5. Aberrant expressions of survivin and GloI were illustrated involvement in progestin resistance4,8,30,31. In addition, disordered signaling pathways were also linked to progestin resistance including abnormally activated PI3K/AKT, Fas/FasL and NRF2 signaling7,9,32,33,34,35. In current study, we found a novel mechanism which is different from previous reports. An enhanced metabolism of progestin mediated by NRF2-TET1-AKR1C1 axis is associated with the lack of response to progestin. The increase expression of TET1, NRF2 and AKR1C1 resulted in converting more therapeutic progestin to less potent metabolite 20α-dihydroxyprogesterone, and might finally lead to the failure of progestin therapy. This kind of drug-resistant mechanism is different with the well-known functions of NRF2, such as increased oxidative stress, enhanced drug efflux or reduced drug uptake36,37,38. In last decades, a majority of studies paid more attention to the ligand and ignored the change of progestin itself39,40,41,42,43. Thus, our current study highlights the variation of progestin metabolism and demonstrated it is also a key target for successful progestin therapy.
Brusatol, as a quassinoid natural product, is widely acknowledged as a potent NRF2 pathway inhibitor showing greater than 50% inhibition of luciferase activity at nanomole concentration, while its general effect on translation appears at higher concentration44. Consistently, we found brusatol could significantly inhibit endometrial cancer cell proliferation at nanomole concentration alone or with MPA. Considering NRF2 as an essential role in progestin resistance and the known inhibition of NRF2 by brusatol in progestin resistance9, we further investigated the underlying detailed mechanism of brusatol regulating progestin metabolism mediated by NRF2. Here, the decline expressions of NRF2, TET1 and AKR1C1 by brusatol may attenuate the catabolism of progestin, which in turn results in enhanced suppression on endometrial cellular growth in the presence of MPA.
Functional analysis revealed that AKR1C1 inactivates progestin by forming 20α-dihydroxyprogesterone, which prompted us to investigate the metabolism of progestin in precancerous endometrial tissues and endometrial cancer. The main function of progestin is to regulate the differentiation of endometrial epithelial cells and to limit cell proliferation45,46. Dysregulation of progestin metabolism may contribute to the formation of endometrial lesions and even lead to the loss of the therapeutic effect of exogenous progestin. Previous study demonstrated that increased expression of AKR1C1 and AKR1C3 in endometriosis not only decreased expression of progesterone receptor B but also induced production of less active metabolite, 20α-dihydroxyprogesterone16. Similarly, this might be one of another mechanism by which NRF2-mediated progestin resistance.
NRF2, a target molecule of brusatol, contributes to drug resistance in a broad spectrum of cancer cell types via the “dark side” effect. Brusatol is a specific inhibitor of NRF2 and enhances NRF2 degradation via a ubiquitination-dependent pathway22. But how it suppresses endometrial cancer cell proliferation via NRF2 has not been clarified. TET1 has been involved in chemoresistance in endometrial cancer18. Here, we found that the TET1 promoter region contains four AREs, and the first ARE was identified as essential for NRF2-mediated regulation of TET1 expression. Thus, TET1 may serve as a novel NRF2 target gene. This is consistent with our previous findings that overexpression of NRF2 elevated TET1 expression18. Moreover, knocking down TET1 enhanced MPA-induced proliferation inhibition in ishikawa-NRF2 cells. This suggests that TET1 is required for NRF2 induced progestin resistance. AKR1C1 has been identified as another NRF2 target gene in previous studies11,12. In this study, we found TET1 is transcriptionally regulated by NRF2 and AKR1C1 is upregulated by NRF2-TET1 via an increase of hydroxymethylation level in the promoter region. However, this kind of epigenetic modification can be erased by brusatol. Briefly, the two different mechanisms of NRF2 regulating AKR1C1 are as follows: (1) direct transcriptional regulation by NRF2 through ARE element located at the AKR1C1 promoter; (2) indirect NRF2-AKR1C1 interaction mediated by hydroxylmethylation by TET1. We speculate the above two mechanisms contribute equally to brusatol attenuating endometrial cancer resistance to progestin by inhibiting NRF2, which remains to be verified in future studies.
We found that brusatol significantly suppressed AKR1C1 expression and blocked AKR1C1-mediated progestin metabolism. In addition, brusatol combined with MPA potently reduced AKR1C1 expression in the human endometrial cancer organoids. Thus, blocking progestin metabolism may be the molecular mechanism by which brusatol sensitizes endometrial cancer cells to progestin. Detection the local metabolism of progestin in endometrial lesion tissue is difficult; however, the expression profile of AKR1C1 may represent the metabolic activity of progestin, to a certain extent. IHC assay indicated that AKR1C1 was strongly expressed in paired tissues from the same individual with progestin resistance regardless pre- or post-progestin treatment. Conversely, in the cases with good response to progestin, high level of AKR1C1 expression disappeared in atrophic glands underwent progestin administration compared with that of pre-treatment. These data indicated that AKR1C1 is a good marker for identifying patients with a poor response to progestin. Interestingly, an inverse expression pattern of AKR1C1 between stromal and glandular epithelial cell has been observed. AKR1C1 is overexpressed in the stromal cells compared with around atrophic glands in patients with a good response to progestin, whereas loss of AKR1C1 is observed in stromal cells compared with the hyperplasia or cancer glands in the patients with progestin resistance. Previous studies demonstrated that endometrial stromal cells contribute to endometrial regeneration, repair and inhibit endometrial epithelial cell growth by secreting growth factors or hormones47,48,49,50,51,52. Therefore, we consider that it is no longer necessary to maintain a high level of progestin to limit glandular epithelial cell excessive growth in the atrophic glands, if it shows a well response to progestin treatment. An increased AKR1C1 expression in stromal cells could guarantee a low level of progestin by enhancing progestin catabolism. By contrast, in the progestin-resistant cases, the lack of progestin catabolism due to loss AKR1C1 in these stromal cells resulted in a high level of progestin to suppress hyperplasia glandular epithelial cellular growth with a compensatory manner.
However, there are some limitations in this work that could be addressed in the future studies. Firstly, brusatol, as a general protein inhibitor, might improve progestin resistance through other non-NRF2 dependent pathways besides our findings. Meanwhile, there may be some other NRF2 specific inhibitors such as Halofugionne53, or unknown compounds potentially promoting progestin effect by NRF2, to be further investigated.
In conclusion, our findings elaborate the mechanisms of brusatol sensitizing endometrial cancer to progestin by suppressing progestin metabolism mediated by NRF2-TET1-AKR1C1 pathway, providing a novel insight into progestin resistance. And we confirm that AKR1C1 may be a useful biomarker for predicting progestin resistance in the treatment of endometrial atypical hyperplasia and endometrial cancer.
Data availability
The data used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Key, T. J. & Pike, M. C. The dose-effect relationship between ‘unopposed’ oestrogens and endometrial mitotic rate: its central role in explaining and predicting endometrial cancer risk. Br J Cancer 57, 205-212 (1988).
Cherry, N., McNamee, R., Heagerty, A., Kitchener, H. & Hannaford, P. Long-term safety of unopposed estrogen used by women surviving myocardial infarction: 14-year follow-up of the ESPRIT randomised controlled trial. BJOG 121, 700-705; discussion 705 (2014).
Chen, X. J., Zhang, Z. B., Feng, Y. J., Fadare, O., Wang, J., Ai, Z. H. et al. Aberrant survivin expression in endometrial hyperplasia: another mechanism of progestin resistance. Modern Pathol 22, 699-708 (2009).
Jiang, Y., Chen, X., Wei, Y., Feng, Y., Zheng, W. & Zhang, Z. Metformin sensitizes endometrial cancer cells to progestin by targeting TET1 to downregulate glyoxalase I expression. Biomed Pharmacother 113, 108712 (2019).
Zhao, S., Chen, X., Lu, X., Yu, Y. & Feng, Y. Epidermal growth factor receptor signaling enhanced by long-term medroxyprogesterone acetate treatment in endometrial carcinoma. Gynecol Oncol 105, 45-54 (2007).
Satyaswaroop, P. G., Clarke, C. L., Zaino, R. J. & Mortel, R. Apparent resistance in human endometrial carcinoma during combination treatment with tamoxifen and progestin may result from desensitization following downregulation of tumor progesterone receptor. Cancer Lett 62, 107-114 (1992).
Gu, C., Zhang, Z., Yu, Y., Liu, Y., Zhao, F., Yin, L. et al. Inhibiting the PI3K/Akt pathway reversed progestin resistance in endometrial cancer. Cancer Sci 102, 557-564 (2011).
Zhang, Z., Dong, L., Sui, L., Yang, Y., Liu, X., Yu, Y. et al. Metformin reverses progestin resistance in endometrial cancer cells by downregulating GloI expression. Int J Gynecol Cancer 21, 213-221 (2011).
Wang, Y., Wang, Y., Zhang, Z., Park, J. Y., Guo, D., Liao, H. et al. Mechanism of progestin resistance in endometrial precancer/cancer through Nrf2-AKR1C1 pathway. Oncotarget 7, 10363-10372 (2016).
Jiang, T., Chen, N., Zhao, F., Wang, X. J., Kong, B., Zheng, W. et al. High levels of Nrf2 determine chemoresistance in type II endometrial cancer. Cancer Res 70, 5486-5496 (2010).
Lou, H., Du, S., Ji, Q. & Stolz, A. Induction of AKR1C2 by phase II inducers: identification of a distal consensus antioxidant response element regulated by NRF2. Mol Pharmacol 69, 1662-1672 (2006).
Jung, K. A., Choi, B. H., Nam, C. W., Song, M., Kim, S. T., Lee, J. Y. et al. Identification of aldo-keto reductases as NRF2-target marker genes in human cells. Toxicol Lett 218, 39-49 (2013).
Rizner, T. L., Smuc, T., Rupreht, R., Sinkovec, J. & Penning, T. M. AKR1C1 and AKR1C3 may determine progesterone and estrogen ratios in endometrial cancer. Mol Cell Endocrinol 248, 126-135 (2006).
Smuc, T. & Rizner, T. L. Aberrant pre-receptor regulation of estrogen and progesterone action in endometrial cancer. Mol Cell Endocrinol 301, 74-82 (2009).
Beranic, N., Brozic, P., Brus, B., Sosic, I., Gobec, S. & Lanisnik Rizner, T. Expression of human aldo-keto reductase 1C2 in cell lines of peritoneal endometriosis: potential implications in metabolism of progesterone and dydrogesterone and inhibition by progestins. J Steroid Biochem Mol Biol 130, 16-25 (2012).
Beranic, N., Gobec, S. & Rizner, T. L. Progestins as inhibitors of the human 20-ketosteroid reductases, AKR1C1 and AKR1C3. Chem Biol Interact 191, 227-233 (2011).
Sharma, K. K., Lindqvist, A., Zhou, X. J., Auchus, R. J., Penning, T. M. & Andersson, S. Deoxycorticosterone inactivation by AKR1C3 in human mineralocorticoid target tissues. Mol Cell Endocrinol 248, 79-86 (2006).
Bai, M., Yang, L., Liao, H., Liang, X., Xie, B., Xiong, J. et al. Metformin sensitizes endometrial cancer cells to chemotherapy through IDH1-induced Nrf2 expression via an epigenetic mechanism. Oncogene 37, 5666-5681 (2018).
Kang, K. A., Piao, M. J., Ryu, Y. S., Kang, H. K., Chang, W. Y., Keum, Y. S. et al. Interaction of DNA demethylase and histone methyltransferase upregulates Nrf2 in 5-fluorouracil-resistant colon cancer cells. Oncotarget 7, 40594-40620 (2016).
Wang, J., Zhang, D., Du, J., Zhou, C., Li, Z., Liu, X. et al. Tet1 facilitates hypoxia tolerance by stabilizing the HIF-α proteins independent of its methylcytosine dioxygenase activity. Nucleic Acids Res 45, 12700-12714 (2017).
Bai, X., Zhang, H., Zhou, Y., Nagaoka, K., Meng, J., Ji, C. et al. TET1 promotes malignant progression of cholangiocarcinoma with IDH1 wild-type. Hepatology 73, 1747-1763 (2021).
Ren, D., Villeneuve, N. F., Jiang, T., Wu, T., Lau, A., Toppin, H. A. et al. Brusatol enhances the efficacy of chemotherapy by inhibiting the Nrf2-mediated defense mechanism. Proc Natl Acad Sci USA 108, 1433-1438 (2011).
Lee, J. H., Rangappa, S., Mohan, C. D., Sethi, G., Lin, Z. X., Rangappa, K. S. et al. Brusatol, a Nrf2 Inhibitor Targets STAT3 Signaling Cascade in Head and Neck Squamous Cell Carcinoma. Biomolecules 9, 550 (2019).
Xiang, Y., Ye, W., Huang, C., Lou, B., Zhang, J., Yu, D. et al. Brusatol inhibits growth and induces apoptosis in pancreatic cancer cells via JNK/p38 MAPK/NF-κb/Stat3/Bcl-2 signaling pathway. Biochem Bioph Res Co 487, 820-826 (2017).
Feng, L., Li, J., Yang, L., Zhu, L., Huang, X., Zhang, S. et al. Tamoxifen activates Nrf2-dependent SQSTM1 transcription to promote endometrial hyperplasia. Theranostics 7, 1890-1900 (2017).
Vartanian, S., Ma, T. P., Lee, J., Haverty, P. M., Kirkpatrick, D. S., Yu, K. et al. Application of Mass Spectrometry Profiling to Establish Brusatol as an Inhibitor of Global Protein Synthesis. Mol Cell Proteomics 15, 1220-1231 (2016).
Liu, Y., Lu, Y., Celiku, O., Li, A., Wu, Q., Zhou, Y. et al. Targeting IDH1-Mutated Malignancies with NRF2 Blockade. J Natl Cancer Inst 111, 1033-1041 (2019).
Oh, E. T., Kim, C. W., Kim, H. G., Lee, J. S. & Park, H. J. Brusatol-Mediated Inhibition of c-Myc Increases HIF-1alpha Degradation and Causes Cell Death in Colorectal Cancer under Hypoxia. Theranostics 7, 3415-3431 (2017).
Turco, M. Y., Gardner, L., Hughes, J., Cindrova-Davies, T., Gomez, M. J., Farrell, L. et al. Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat Cell Biol 19, 568-577 (2017).
Chen, X., Zhang, Z., Feng, Y., Fadare, O., Wang, J., Ai, Z. et al. Aberrant survivin expression in endometrial hyperplasia: another mechanism of progestin resistance. Mod Pathol 22, 699-708 (2009).
Ai, Z., Yin, L., Zhou, X., Zhu, Y., Zhu, D., Yu, Y. et al. Inhibition of survivin reduces cell proliferation and induces apoptosis in human endometrial cancer. Cancer 107, 746-756 (2006).
Wang, S., Pudney, J., Song, J., Mor, G., Schwartz, P. E. & Zheng, W. Mechanisms involved in the evolution of progestin resistance in human endometrial hyperplasia--precursor of endometrial cancer. Gynecol Oncol 88, 108-117 (2003).
Shan, W., Wang, C., Zhang, Z., Gu, C., Ning, C., Luo, X. et al. Conservative therapy with metformin plus megestrol acetate for endometrial atypical hyperplasia. J Gynecol Oncol 25, 214-220 (2014).
Xie, B. Y., Lv, Q. Y., Ning, C. C., Yang, B. Y., Shan, W. W., Cheng, Y. L. et al. TET1-GPER-PI3K/AKT pathway is involved in insulin-driven endometrial cancer cell proliferation. Biochem Bioph Res Co 482, 857-862 (2017).
Travaglino, A., Raffone, A., Saccone, G., Insabato, L., Mollo, A., De Placido, G. et al. Immunohistochemical predictive markers of response to conservative treatment of endometrial hyperplasia and early endometrial cancer: A systematic review. Acta Obstet Gyn Scan 98, 1086-1099 (2019).
Kensler, T. W., Wakabayashi, N. & Biswal, S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol 47, 89-116 (2007).
Zhang, D. D. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev 38, 769-789 (2006).
Hayes, J. D. & McMahon, M. NRF2 and KEAP1 mutations: permanent activation of an adaptive response in cancer. Trends Biochem Sci 34, 176-188 (2009).
Li, Y., Huang, C., Kavlashvili, T., Fronk, A., Zhang, Y., Wei, Y. et al. Loss of progesterone receptor through epigenetic regulation is associated with poor prognosis in solid tumors. Am J Cancer Res 10, 1827-1843 (2020).
Rodriguez, M. I., Warden, M. & Darney, P. D. Intrauterine progestins, progesterone antagonists, and receptor modulators: a review of gynecologic applications. Am J Obstet Gynecol 202, 420-428 (2010).
Kim, J. J. & Chapman-Davis, E. Role of progesterone in endometrial cancer. Semin Reprod Med 28, 81-90 (2010).
Janzen, D. M., Rosales, M. A., Paik, D. Y., Lee, D. S., Smith, D. A., Witte, O. N. et al. Progesterone receptor signaling in the microenvironment of endometrial cancer influences its response to hormonal therapy. Cancer Res 73, 4697-4710 (2013).
Lee, II, Maniar, K., Lydon, J. P. & Kim, J. J. Akt regulates progesterone receptor B-dependent transcription and angiogenesis in endometrial cancer cells. Oncogene 35, 5191-5201 (2016).
Zhang, D. D. & Chapman, E. The role of natural products in revealing NRF2 function. Nat Prod Rep 37, 797-826 (2020).
Mote, P. A., Balleine, R. L., McGowan, E. M. & Clarke, C. L. Colocalization of progesterone receptors A and B by dual immunofluorescent histochemistry in human endometrium during the menstrual cycle. J Clin Endocrinol Metab 84, 2963-2971 (1999).
Yang, S., Fang, Z., Gurates, B., Tamura, M., Miller, J., Ferrer, K. et al. Stromal PRs mediate induction of 17beta-hydroxysteroid dehydrogenase type 2 expression in human endometrial epithelium: a paracrine mechanism for inactivation of E2. Mol Endocrinol 15, 2093-2105 (2001).
Terzaghi-Howe, M. & McKeown, C. Inhibition of carcinogen-altered rat tracheal epithelial cells by normal epithelial cell-conditioned medium. Cancer Res 46, 917-921 (1986).
Terzaghi-Howe, M. Inhibition of carcinogen-altered rat tracheal epithelial cell proliferation by normal epithelial cells in vivo. Carcinogenesis 8, 145-150 (1987).
Arnold, J. T., Kaufman, D. G., Seppala, M. & Lessey, B. A. Endometrial stromal cells regulate epithelial cell growth in vitro: a new co-culture model. Hum Reprod 16, 836-845 (2001).
Uchida, H., Maruyama, T., Nagashima, T., Asada, H. & Yoshimura, Y. Histone deacetylase inhibitors induce differentiation of human endometrial adenocarcinoma cells through up-regulation of glycodelin. Endocrinology 146, 5365-5373 (2005).
Shi, M., Zhang, H., Li, M., Xue, J., Fu, Y., Yan, L. et al. Normal endometrial stromal cells regulate survival and apoptosis signaling through PI3K/AKt/Survivin pathway in endometrial adenocarcinoma cells in vitro. Gynecol Oncol 123, 387-392 (2011).
Yin, M., Zhou, H. J., Lin, C., Long, L., Yang, X., Zhang, H. et al. CD34(+)KLF4(+) Stromal Stem Cells Contribute to Endometrial Regeneration and Repair. Cell Rep 27, 2709-2724 (2019).
Tsuchida, K., Tsujita, T., Hayashi, M., Ojima, A., Keleku-Lukwete, N., Katsuoka, F. et al. Halofuginone enhances the chemo-sensitivity of cancer cells by suppressing NRF2 accumulation. Free Radical Bio Med 103, 236-247 (2017).
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (grants 81872111 and 81672562), National Key Technology R&D Program of China (2019YFC1005200 and 2019YFC1005201), Shanghai Municipal Science and Technology Committee of Shanghai outstanding academic leaders plan (19XD1423100), the project of Outstanding Medical Doctor for Z.Z., Shanghai Municipal Education Commission—Gaofeng Clinical Medicine Grant Support (20181714). We thank all members in Z.Z. lab.
Author information
Authors and Affiliations
Contributions
Z.Z., S.W. and J.L. performed study concept and designed the study. M.H., D.S. and J.Y. performed most of the experiments and wrote the manuscript. Y.F., Z.Q., B.H. and Q.Z. performed part of the experiments. M.H., D.S., J.Y., Y.F., Z.Q., B.H. and Q.Z. analyzed and interpreted the data. X.C., Y.W., H.Z., Y.W., Y.F. and W.Z. provided technical and material support. Z.Z. and J.L. revised the manuscript with comments from all authors. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Human endometrial hyperplasia and cancer tissue samples were obtained from Shanghai General Hospital Affiliated to Shanghai Jiao Tong University and Shanghai First Maternity and Infant Hospital Affiliated to Tongji University School of Medicine. Patients’ informed consent was obtained. All animal studies were approved by the Animal Ethics Committee of Shanghai General Hospital experimental protocols.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, M., Sun, D., Yu, J. et al. Brusatol sensitizes endometrial hyperplasia and cancer to progestin by suppressing NRF2-TET1-AKR1C1-mediated progestin metabolism. Lab Invest 102, 1335–1345 (2022). https://doi.org/10.1038/s41374-022-00816-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41374-022-00816-5