Abstract
Macrophage polarization mediates the development of inflammatory diseases. However, the polarization status at various stages of gout is not fully understood. Our study aimed to define the evolution of macrophage polarization in acute and chronic gout. Normal human synovium and synovium with tophi were collected for immunofluorescence (IF). Rat gouty joints were collected for joint thickness assessment and pathological evaluation. Tissue mRNA expression of inducible nitric oxide synthase (iNOS) and arginase-1 (Arg-1) were evaluated. Mouse peritoneal macrophages and THP-1 derived macrophages were stimulated by monosodium urate (MSU) crystals and were collected for detection of interleukin (IL) -1β and IL-37 levels and iNOS/Arg-1 ratio. Arg-1 and IL-37 were highly expressed in normal synovium and synovium with tophi. In rat gouty joints, the inflammatory cell counts and ankle thickness began to increase at 2 h, peaked at 24 h, and was decreased spontaneously. An increase in macrophages preceded the neutrophils infiltration. Infiltration of M1 was positively related with the severity of arthritis. M2 appeared in an early stage (at 2 h) of inflammation. The number of M1 macrophages was comparable to that of M2 from 2 to 12 h and exceeded M2 number at 18 h and 24 h. The ratios of M2/M1 reversed at 48 h and remained reversed until 120 h. In mice gouty joints, iNOS/Arg-1 mRNA ratio was significantly higher than the that in control group at 8 h. The proportion of neutrophils and M1-macrophages reached peak at 4 h in mice model with peritoneal gout. Concentration of IL-1β and ratio of iNOS/Arg-1 were increased at 6 h, peaked at 48 h, and were then decreased at 72 h in vitro, while the concentration of IL-37 peaked at 2 h and then decreased. In summary, altered macrophage polarization was observed in various stages of gouty inflammation. Macrophages in acute gout were polarized into M1 at early stage and into M2 at later stage while the macrophages in chronic gout mainly were only polarized towards M2. The number of M1 rose with the progression of inflammation. Early increase of M2 was observed, which might be generated directly from M0.
Similar content being viewed by others
Introduction
Acute gouty arthritis is a self-limiting innate in response to the deposition of monosodium urate (MSU) crystals in joint and surrounding soft tissue. It is commonly known that MSU crystals can trigger gout by promoting inflammation through resident macrophages and activating immune cells that lead to the release of pro-inflammatory mediators (including IL-1β) and chemokines that drive the rapid recruitment of classical innate inflammatory cells, primarily neutrophils and monocytes1. Monocytes play multiple roles in acute immune response, such as replenishing resident macrophages under normal conditions; moving quickly in response to inflammatory signals to the inflammatory sites in the tissues; and dividing or differentiating into macrophages to elicit an immune response. There is a growing body of evidence indicating that macrophages play a pivotal role not only in the initiation but also in the progression and resolution of acute gouty inflammation2.
Macrophages are usually subdivided into M1 and M2, depending on their roles in immune response. The macrophage polarization is increasingly recognized as an important pathogenetic factor in inflammatory and neoplastic diseases. Macrophages that encourage inflammation are called M1, whereas those that decrease inflammation and encourage tissue repair are called M23,4. This difference is reflected in their metabolism; M1 macrophages have the unique ability to metabolize arginine to the “killer” molecule nitric oxide (NO), whereas M2 macrophages have the unique ability to metabolize arginine to the “repair” molecule ornithine. To note, the molecules primarily responsible for these “Fight” (NO) or “Fix” (ornithine) activities both arise from arginine via enzymatic pathways (iNOS and Arg-1) that down regulate each other5. M1 and M2 macrophages are distinguished by the differential expression of diverse molecules, e.g., iNOS, metalloproteinases and arginase-16,7.
Although studies of pathogenesis of gout are ample, in vivo quantitation of inflammatory cells and analysis of macrophage polarization are rarely reported. Macrophage polarization states in different gouty inflammation stages (initiation, progression and resolution) have not been well investigated. In addition, studies focusing on macrophage polarization are mostly performed in vitro and do not reflect the complexity of immune responses observed in vivo8. Thus, in situ characterization of macrophage polarization is of great importance. Our studies aimed to determine the types and quantity of inflammatory cells (neutrophils, macrophages) and to define the evolution of macrophage polarization in vivo and in vitro.
Both IL-37 and IL-1β can be activated by caspase-1, while IL-37 has the opposite function to IL-1β9. Our preliminary studies have demonstrated that MSU activates NOD-like receptor thermal protein domain associated protein 3 (NALP3) inflammasome to produce IL-37, which initiates the remission of acute inflammation10. In this study, we observed the changes in IL-1β and IL-37 in addition to macrophage polarization.
Materials and methods
Patients and specimen collection
The tophi specimen in synovium from a patient aged 50 years old with chronic gout (involving the knee joint) and the synovium specimen from a patient aged 48 years old without medical history of gout were collected after knee arthroscopy in the Department of Sports Medicine, Huashan Hospital, Fudan University. All samples were obtained with the patient’s written informed consent and approved by Ethics Committee of Huashan Hospital.
Preparation of MSU crystal
MSU crystals were prepared as described by Denko and Whitehouse11. In short, 4 g uric acid was dissolved in 800 mL of deionized water, heated to 60 °C, adjusted to pH 8.9 with 0.5 N NaOH, and crystallized overnight at room temperature. MSU crystals were recovered by centrifugation, washed with distilled water and dried at 40 °C for 24 h. Crystal shape and birefringence were assessed by compensated polarized light microscopy. MSU crystals were milled and then sterilized by heating at 180 °C for 2 h before each experiment. Less than 0.015 EU/ml endotoxin were measured in MSU crystal preparations by Limulus amebocyte lysate assay (Sigma-Aldrich, St. Louis, MO, USA).
Animals
Seven to eight-week Wistar male rats and C57B/6L mice obtained from Jiesijie Laboratory Animal Limited Corporation (Shanghai, China) were housed at 24 ± 2 °C under 12-h light/12-h dark cycles in a pathogen-free facility at Central Institute for Experimental Animals of Shanghai Medical School. Animal experiments were conducted according to protocols approved by the Animal Committee of Fudan University. Rats and mice were anesthetized in a plastic cage by inhalation of 3% isoflurane (Sangin Biotech, Shanghai, China) and then subjected to intra-articular injection of 50 μL (1 mg) MSU suspension into the right ankle. Phosphate-buffered saline (PBS) was simultaneously injected into the left ankle as internal control. Rats and mice were sacrificed under CO2 anesthesia and joint samples were collected at several time points (0, 2, 4, 6, 12, 18, 24, 48, 72, 96, 120, 144, 168 and 240 h in rats; 0 and 8 h in mice) after intra-articular injection of MSU, respectively. Joint swelling degree was calculated by measuring ankle thickness using digital vernier caliper12. Joint inflammation was expressed as the ratio of the joint thickness of the inflamed over the normal control joint. To build a mouse model of acute gouty peritonitis, MSU crystals suspended in PBS (3 mg/0.5 ml) were injected intraperitoneally.
Culture of THP-1 cells and intervention of MSU
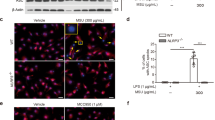
THP-1 cells were obtained from National Collection of Authenticated Cell Cultures (Shanghai, China) and were cultured with RPMI Medium 1640 + 10% FBS (both from Thermo Fisher Scientific, MA, USA) in 5% CO2, at 37 °C. A total of 100 nmol/L polymethacrylate (PMA) (Sigma-Aldrich) was added to the culture plate for 48 h to prime THP-1 cells to macrophages, and cell attachment and pseudopodia growth were detected under microscope as observation indicators. To obtain MSU-treated THP-1 cells in vitro, 100 μg/mL MSU was added into THP-1 derived macrophages for 48 h.
Histopathology and Immunohistochemistry
Animals were humanely euthanized, and joint samples were collected and freshly frozen for RNA isolation or fixed in 3.5% formaldehyde. The fixed samples were embedded in paraffin, sectioned at 5 μm in thickness, and were routinely stained with hematoxylin-eosin (HE) for morphological evaluation.
For immunohistochemical staining, sections were rehydrated. After antigen retrieval using citrate buffer (10 mM, pH 6), sections were blocked with 5% BSA in PBS and stained with anti-F4/80 antibody and anti-CD14 antibody (all form Santa Cruz Biotechnology, Dallas, TX, USA) overnight at 4 °C. Signals were detected using ImmunoCruz rabbit™ LSAB Staining System (Santa Cruz Biotechnology). Finally, the sections were counterstained with 20% hematoxylin. The slides were dehydrated, cleared and evaluated. Each sample was incubated with an isotypic antibody dilution under the same experimental conditions as the negative control.
Double staining immunofluorescence (IF)
To identify the polarization states of macrophages (M1 co-expressing F4/80/CD14 + iNOS and M2 co-expressing F4/80/CD14 + Arg-1), we performed double or triple IF staining. Non-specific staining was blocked with 10% normal goat serum in PBS containing 0.3% Triton X100 for 30 min and sections were incubated overnight at 4 °C with primary antibodies: anti-F4/80 antibody, anti-CD14 antibody, anti-iNOS antibody and anti-Arg-1 antibody (all from Santa Cruz Biotechnology). Sections were then incubated with secondary antibodies: goat anti-rabbit IgG H&L Alexa Fluor® 488 conjugated antibody, goat anti-mouse IgG H&L Alexa Fluor® 647 conjugated antibody and goat anti-mouse IgG H&L Cy3® antibody (all from Abcam, Boston, MA, USA) at room temperature for 1 h. All slides were mounted with the mounting medium, including 4’, 6-diamino-2-phenylindole (DAPI; Abcam).
Analysis of inflammatory cell type
Neutrophils and macrophages were counted during the semi-quantitative evaluation. Neutrophils were identified by morphology based on HE staining, while macrophages were identified by positive F4/80 immunostaining. Areas with high cellular density were selected, and two trained pathologists independently counted each category of cells in five selected areas of high power fields (HPF) at 400× magnification. The final cell numbers were calculated by averaging the counts from individual pathologists. Images were acquired using a microscope (Olympus BX50, Olympus, Tokyo, Japan) with a 40× objective and the analysis image processing software (Olympus). The field of view under 40× objective is 0.22 mm2.
For IF, the analysis was performed using epifluorescent (Olympus). For the analysis of M1 and M2 phenotypes, semi-quantification of F4/80+ and iNOS+ or Arg-1+ cells was performed at all 13 time points of gouty inflammation in rats and mice. M1 and M2 cells were counted by experienced researcher. A few other cells expressing either iNOS or Arg1 were also detected, which showed F4/80−, and thus no further histological analysis was performed. Double-labeled cells were quantified in 5 randomly selected areas (400× magnification, each 0.22 mm2). The ratios of M1/M2 were also calculated.
Quantitative Real-Time Polymerase Chain Reaction (RT-PCR)
Total RNA was extracted from MSU-treated joint samples at 10 time points and PBS-treated joint samples (control samples), and the final concentration of RNA was adjusted to 0.5 mg/mL. cDNA was synthesized using the Rever TraAceHqPCR RT Kit (TOYOBO, Osaka, Japan). Quantitative real-time PCR was performed on a 7500 Fast Real-Time PCR System (AB Applied Biosystems, USA) using SYBR Green Realtime PCR Master Mix (TOYOBO). The specificity of amplification was assessed for each sample by melting curve analysis. Relative quantification was performed using standard curve analysis. The quantification data are presented as the ratio to the control level. The rat gene-specific primers used were as follows: iNOS, 5’- GATTTTTCACGACACCCTTCACC-3’ (forward) and 5’- GGTCCTCTGGTCAAACTCTTGGAG-3’ (reverse); Arg-1, 5’- TGAACCCAACTCTTGGGAAG-3’(forward) and 5’- GTGATGCCCCAGATGACTTT-3’ (reverse); GAPDH, 5’- TGCTGGTGCTGAGTATGTCGTG-3’ (forward) and 5’- CGGAGATGATGACCCTTTTGG-3’ (reverse).
ELISA
Culture supernatant was harvested from MSU-treated THP-1 cells and ELISA was conducted according to the manufacturer’s instructions (Anogen Biotech Laboratories Ltd., Mississauga, Ontario, Canada).
Flow cytometry detection
Flow cytometry was used to detect the expression of iNOS/Arg-1 and to distinguish neutrophils and macrophages. The anti-iNOS antibody, anti-Arg1 antibody, anti-F4/80 antibody and anti-Ly6G antibody (Thermo Fisher Scientific, MA, USA) were used to mark intracellular proteins by means of eBioscience Permeabilization Buffer (Thermo Fisher Scientific) according to the manufacturer’s instructions. The flow cytometry was performed using the Accuri C6 (BD Biosciences, San Diego, CA, USA).
Statistical evaluation
Data are presented as mean ± S.E.M. and analyzed by GraphPad Prism 5.01 program (GraphPad Software, La Jolla, CA, USA). Significant difference was assessed by the Student’s t test or Mann–Whitney test (between two comparison groups) for non-parametric measures. Repeated measures analysis of variance (ANOVA) followed by S-N-K test was applied for post-hoc analysis of differences between groups. The Spearman test was used for correlation analysis. A value of P < 0.05 was considered significant.
Results
Macrophage polarization in normal human synovium and synovium with tophi from patient with gout
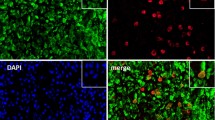
The expression of Arg-1 was higher than iNOS in both normal synovium and synovium with tophi (Fig. 1A, B). Intriguingly, iNOS was mainly expressed in regions of amorphous matter formed by dissolving MSU in tophi. IL-37 was highly expressed in both normal synovium and synovium with tophi, while IL-1β was only highly expressed in synovium with tophi (Fig. 2A, B).
Pathological assessment of inflammation and quantitation of inflammatory cells in rat gouty joints
The injection of MSU crystals induced an acute inflammation in rats and led to joint swelling. The ankle thickness was increased at 2 h, reached peak at 24 h, and was then decrease spontaneously over the next 9 days, simulating the clinical course of human acute self-limited gout (Fig. 3A). In addition, total inflammatory cell count reflected the clinical course of spontaneous resolution of acute gout (Fig. 3B).
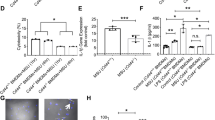
A Joint thickness was measured at 0, 2, 4, 6, 12, 18, 24, 48, 72, 96, 120, 168 and 240 h after MSU administration. B Kinetics of inflammatory cells in rat gouty arthritis. C Kinetics of neutrophils and macrophages in joint tissue from gouty arthritis samples of rats. D Pathological manifestation of inflammatory evolution during gouty arthritis course of rat from onset to remission (HE staining). E Pathological manifestation of F4/80+ macrophages infiltration at different time points during gouty inflammation of rat by IHC. n = 3 mice per group. P values significantly different from 0 h: *P < 0.05, **P < 0.01, ***P < 0.001. Error bars indicate standard error of mean (SEM). MSU monosodium urate, HE hematoxylin-eosin, IHC immunohistochemistry.
HE- and IHC-stained sections revealed significant increase in inflammatory cells, including highlighted F4/80 positive macrophages and neutrophils that were infiltrated in the edematous synovium, joint space and surrounding soft tissues at 2 h to 24 h. The cell numbers peaked at 24 h and thereafter showed decreasing trend until up to 240 h in all samples under transmitted light microscopy (Fig. 3D, E).
An increase in macrophages preceding the neutrophils infiltration was observed. Macrophages were increased at 2 h, whereas neutrophils were increased at 4 h after MSU injection (Fig. 3C).
Macrophage immune-phenotypes and kinetics of M1 and M2 macrophages in rat gouty arthritis
Activated macrophages in joint were identified by F4/80 marker. Activated F4/80 cells could express iNOS (M1 marker) or Arg-1 (M2 marker) (Fig. 4A), or neither of them (non-M1, non-M2) (Figure not shown). Similar to the kinetics of inflammatory cells, the M1 cell number began to increase at 2 h, reached the highest value at 24 h, and then was decreased until up to 240 h (Fig. 4B). To our surprise, M2 appeared at an early stage (2 h after MSU injection) and reached peak level at 48 h (Fig. 4B). By 2–12 h, the numbers of M1 and M2 were comparable, followed by M1 cell counts exceeding M2 at 18 and 24 h However, the M2 cells outnumbered M1 thereafter; the ratios of M2/M1 reversed at 48 h and remained unchanged reversed until 120 h (Fig. 4B).
A Gouty arthritis sections after 12 h of MSU injection were immunolabeled by anti-F4/80 antibody and visualized with a secondary antibody conjugated with Alexa Fluor® 488 (green fluorochrome) and captured by fluorescence microscope at 200x magnification. M1 (iNOS) and M2 (Arg-1) cytoplasmic markers were labeled by antibodies and visualized with a secondary antibody conjugated with Alexa Fluor® 647 (red fluorochrome). Merged images showed double fluorescence-labeled cells (F4/80+iNOS+ and F4/80+Arg-1+, respectively) which were turned to orange. B Kinetics of M1 and M2 macrophages in joint tissue from gouty arthritis samples of rats. C Tissue mRNA expression levels of iNOS and Arg-1 at different time points in samples from rat gouty arthritis. iNOS, inducible nitric oxide synthase; Arg-1, arginase-1.
mRNA expression of iNOS in the tissue was positively correlated with M1 counts, whereas mRNA expression of Arg-1 showed 2 peaks, one at 48 h and the other at 120 h, which was partially correlated with M2 counts (Fig. 4C).
Macrophage polarization in mouse models of gout
After MSU was injected into the peritoneal cavity of mice, the peritoneal fluid was collected and centrifuged to enrich the cells. Flow cytometry showed that the proportion of neutrophils reached the highest level at 4 h while the proportion of macrophages was at the lowest level at 4 h (Fig. 5A, B). The iNOS+/Arg-1+ MFI ratio also peaked at 4 h, showing a similar trend to neutrophils proportion (Fig. 5C–E).
A The gating strategy of C–E. B The flow chart of E. C, D The proportion of macrophages and neutrophils in peritoneal lavage fluid. E The MFI ratio of iNOS/Arg-1 in peritoneal macrophages. n = 3 mice per group. P values significantly different from control group: *P < 0.05; **P < 0.01; ***P < 0.001. Error bars indicate standard error of mean (SEM). M macrophages, N neutrophils, MFI median fluorescence intensity, iNOS inducible nitric oxide synthase, Arg-1 arginase-1.
MSU was injected into the right posterior paws of the mice, and the left posterior paws was injected with PBS. The ratio of iNOS+/Arg-1+ in F4/80+ macrophages and the joint thickness were much higher at 8 h after MSU injection (Fig. 6C–F) compared with 0 h (Fig. 6A, B).
A–D Gouty arthritis sections were immunolabeled with anti-F4/80 antibody and visualized with a secondary antibody conjugated with Alexa Fluor ® 647 (red fluorochrome). M1 (iNOS) and M2 (Arg-1) cytoplasmic markers were labeled by the primary antibodies and then visualized using secondary antibodies conjugated with Alexa Fluor® 488 (green fluorochrome). Merged images showed double fluorescence-labeled cells (F4/80+iNOS+ and F4/80+Arg-1+, respectively). E The ratio of iNOS+/Arg-1+ was calculated by counting co-expressed cells. F Joint thickness was tested at 0 and 8 h after MSU administration. n = 3 mice per group. ***P < 0.001. Error bars indicate standard error of mean (SEM). iNOS inducible nitric oxide synthase, Arg-1 arginase-1, MSU monosodium urate.
Macrophage polarization in MSU-induced THP-1 derived macrophages
Concentration of IL-1β and MFI ratio of iNOS+/Arg-1+ were increased at 6 h, peaked at 48 h, and then were decreased at 72 h in vitro (Fig. 7A, B), while the concentration of IL-37 peaked at 2 h and then decreased (Fig. 7C).
A The MFI of iNOS+/Arg-1+ was detected at 3, 6, 9, 12, 24, 48 and 72 h. B, C showed the concentration of IL-1β and IL-37 at 0, 2, 6, 18, 24, 48 and 72 h. Error bars indicate standard error of mean (SEM). *P < 0.05; **P < 0.01; ***P < 0.001. MSU monosodium urate, MFI median fluorescence intensity, iNOS inducible nitric oxide synthase, Arg-1 arginase-1, IL interleukin.
Discussion
Despite extensive studies on gouty patients and animal models, in vivo kinetics of inflammatory cells of gout, especially neutrophils and macrophages has not been well investigated. In this study, we used a rat model of gouty arthritis to explore neutrophils and macrophages evolution and to profile the differentiation pathway of MSU crystal-recruited monocytes in vivo, so as to determine whether the infiltrating macrophages population differentiates into a proinflammatory (M1-like) or anti-inflammatory (M2-like) phenotype. In addition, this study also investigated polarization of macrophages in gouty patients, mice and THP-1 derived macrophages and its association with inflammation severity. In this study, the macrophage polarization in chronic gout was described on the basis of pathological staining of synovium with tophi, while rat, mouse, and THP-1 cell models reflected the macrophage polarization in acute gout. The peaks of inflammation in gouty model of arthritis and peritonitis and MSU-stimulated THP-1 cells were defined by ankle thickness, neutrophil infiltration and concentration of cytokines in peritoneal fluid respectively.
Historically, neutrophils have been widely viewed as the major driving force for orchestrating inflammation in and resolution of gout attacks. Neutrophil influx into the synovium and joint fluid is the pathologic hallmark of acute gout, and these cells are thought to contribute to the pathogenesis of gout via producing inflammatory mediators (including chemokines and superoxide), whereas neutrophil apoptosis and clearance by phagocytic cells are essential components of TGF-β1 production and the resolution of inflammation13,14,15. Our findings on rats consistently supported above conclusions from the point of the pathology, i.e., neutrophils infiltrated in the edematous synovium, joint space and surrounding soft tissues at 4–24 h after the induction of inflammation, the cell amount peaked at 24 h and then decreased spontaneously over the next 9 days, which simulated the clinical course of acute self-limited gout. The ankle swelling index (ankle thickness) followed a parallel course above. An increase in macrophages preceding the neutrophils infiltration was observed, which supported the theory that macrophages are responsible for initiating and promoting inflammation, and the influx of neutrophils is driven by the NLRP3 inflammasome/IL-1β signaling. In a study conducted by Amaral et al., they injected a CXCR2 inhibitor into the knee joints of mice, which prevented MSU crystal-induced influx of neutrophils, indicating that the influx of neutrophils was CXCR2-dependent. Moreover, the study also clearly showed that production of CXCR2-active chemokines (CXCL1 and CXCL2) was NLRP3 inflammasome-dependent16.
The growing evidence of functional heterogeneity of macrophages in different pathologies raises questions of their role in initiating, expanding and spontaneous remission of acute gouty inflammation. Our study on rats showed a significant increase in highlighted F4/80 positive macrophages across the entire gouty inflammatory process from 2 to 24 h, where the number of macrophages was increased and peaked at 24 h and then showed a decreasing trend until up to 240 h. It has been proposed that the self-limiting nature of an acute gout attack is linked to the differentiation state of the MSU crystal–recruited monocyte population. In vitro studies indicated that in response to MSU crystal stimulation, isolated blood monocytes can drive the inflammation, while differentiated macrophages would switch from producing proinflammatory cytokines to producing the anti-inflammatory cytokine TGF-β1 and thus resolve inflammation17,18. However, some other studies have demonstrated that both primary and bone marrow-derived macrophages play a key role in the initiation of the gouty inflammatory cascade19,20,21. In a peritoneal murine model of gout, Martin et al.22 indicated that during the early stages of MSU crystal-induced inflammation, the newly recruited monocyte population exhibited a noninflammatory M0-like phenotype, which could differentiate into proinflammatory M1-like phenotype. However, M2-like phenotype is hard to find.
Together, these findings raise questions about the true functional phenotypes expressed by MSU crystal–recruited macrophages populations in vivo. Based on our results on rats, activated F4/80 cells expressed either iNOS or Arg1 or neither of them (nonM1, nonM2). The M1 cell number began to increase at 2 h, reached the highest level at 24 h, and then was decreased until up to 240 h. Surprisingly, contrary to the findings of previous in vitro and in vivo studies, our results indicate that M2 appeared in an early stage (2 h after MSU injection) and peaked at 48 h. This phenomenon was also seen in our mouse model of peritoneal gouty, where the proportion of Arg-1-dominant macrophages was higher than iNOS-dominant macrophages before 4 h. Although early appearance of M2 was also observed in in vitro experiments, this cannot be used as an argument because the THP-1 cell line used was biased towards M2 polarization per se. The differentiation of macrophages into M2 macrophages in the early stage may also play an important role in directing the resolution of inflammation and tissue repairing in gout. However, a huge array of environmental factors (including cytokines, chemokines, pattern recognition receptors, hormones) differentially regulates macrophage response patterns, resulting in the display of numerous distinct, functional phenotypes. Upon stimulation, a macrophage does not display just a single set of functions but rather displays a progression of functional changes in response to the progressive changes in its microenvironment23. Thus, we speculated that these M2-macrophages in early stage of inflammation might be generated directly from M0, depending on the microenvironment. In-depth study is warranted to reveal underlying mechanism.
In our rat study, the number of M1 was comparable to that of M2 at 2–12 h, while the former exceeded the latter at 18 and 24 h. However, M2 outnumbered M1 thereafter, the ratios of M2/M1 reversed at 48 h and remained reversed until 120 h. mRNA expression of iNOS in the tissue was correlated with M1 counts, whereas mRNA expression of Arg-1 showed two peaks, one at 48 h and the other at 120 h, which were partially correlated with M2 counts. The Arg-1 mRNA level reached a peak at 120 h, while the number of M2 macrophages labeled by F4/80+Arg-1+ and detected by IF did not show the same trend at this time point, because the mRNA level represented Arg-1 transcription level in the entire paw tissue; in addition to macrophages, there are many other cells constitutively expressing Arg-1. Thus, in Fig. 4A, it can also be seen that not all Arg-1+ cells expressed F4/80. CD14+ macrophages were higher in human synovium with tophi than in normal synovium (Supplementary Fig. 1), and Arg-1 was highly expressed than iNOS. Both IL-37 and IL-1β were highly expressed. Interestingly, there were some amorphous areas in synovium with tophi, which were wrapped by collagen fibers, without cells and DNA, and contained MSU crystals, IL-1β and TNF-α24. In our study, iNOS in synovium with tophi was mainly represented in those areas. We speculated that those iNOS may be produced by inflammatory cells which died early and were attached to MSU crystals. The MSU crystals were dissolved during pathological specimen preparation and thus these proteins were retained. This study is limited in that we could not collect synovial tissue from patients with acute gout attacks. Our previous study showed that IL-37, a natural innate immunological suppressor, efficiently reshaped gouty macrophages into non-inflammatory phenotype25, which may explain the quiescence in chronic gout patients with tophi. In mouse models of gout, preponderance of M1 phenotype was accompanied by higher joint thickness and neutrophiles influx. The dominant transformation of M1 in in vitro experiments was accompanied by an increase in the concentration of IL-1β and a decrease in the concentration of IL-37. Together, these findings verified our previous assumption that the infiltration of M1- or M2-macrophages can separately promote and maintain or alleviate and resolve gouty inflammation. In addition, M1/M2 transformation in acute gout which accompanied by a natural remission of inflammation may contribute to the self-limiting process of gout. The clearly opposing functions of the M1- and M2-macrophage phenotypes highlight the need to provide a physiologically relevant immune environment for the investigation of macrophages differentiation, and emphasize the importance of investigating the differentiation of MSU crystal–recruited macrophages in vivo.
The results of this study unveil a new level of complexity in our understanding of the macrophage polarization in gouty arthritis. In conclusion, macrophage polarization is a key factor in acute gout. Our studies verified the previous theory that macrophages can not only initiate but also promote and resolve acute gouty inflammation. Altered macrophage polarization was observed in different stages of acute gout attack. The imbalance of M1/M2 macrophage polarization as a key factor of inflammation severity is confirmed in our study by pathological analysis. Characterization of macrophage polarization into different functional phenotypes is valuable for further investigation of gout pathogenesis, as well as for a better disease management. Our understanding of the mechanism of macrophage polarization is far from complete. The underlying mechanisms of macrophage polarization in gout remain to be identified in future studies.
In summary, altered macrophage polarization was observed in different stages of gouty arthritis in human, rats and mice, and also in in vitro MSU-induced THP-1 derived macrophages. In acute gout, M1 macrophages increased with the progression of inflammation, while M2 macrophages showed an increase as the inflammation resolved in later stage. M2 macrophages in early stage might be generated directly from M0, which depends on the microenvironment. In chronic gout, macrophages mainly polarized towards M2 for inflammatory quiescence.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Dalbeth N, Gosling AL, Gaffo A, Abhishek A. Gout. Lancet 397, 1843–1855 (2021).
Martin WJ, Walton M, Harper J. Resident macrophages initiating and driving inflammation in a monosodium urate monohydrate crystal-induced murine peritoneal model of acute gout. Arthritis Rheum 60, 281–289 (2009).
Funes SC, Rios M, Escobar-Vera J, Kalergis AM. Implications of macrophage polarization in autoimmunity. Immunology 154, 186–195 (2018).
Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8, 958–969 (2008).
Mills CD. M1 and M2 macrophages: oracles of health and disease. Crit Rev Immunol 32, 463–488 (2012).
Orecchioni M, Ghosheh Y, Pramod AB, Ley K. Macrophage polarization: different gene signatures in M1(LPS+) vs. classically and M2(LPS-) vs. alternatively activated macrophages. Front Immunol 10, 1084 (2019).
Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41, 14–20 (2014).
Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122, 787–795 (2012).
Cavalli G, Dinarello CA. Suppression of inflammation and acquired immunity by IL-37. Immunol Rev 281, 179–190 (2018).
Liu L, Xue Y, Zhu Y, Xuan D, Yang X, Liang M, et al. Interleukin 37 limits monosodium urate crystal-induced innate immune responses in human and murine models of gout. Arthritis Res Ther 18, 268 (2016).
Denko CW, Whitehouse MW. Experimental inflammation induced by naturally occurring microcrystalline calcium salts. J Rheumatol 3, 54–62 (1976).
Post AM, Katsikis PD, Tait JF, Geaghan SM, Strauss HW, Blankenberg FG. Imaging cell death with radiolabeled annexin V in an experimental model of rheumatoid arthritis. J Nucl Med 43, 1359–1365 (2002)..
Németh T, Sperandio M, Mócsai A. Neutrophils as emerging therapeutic targets. Nat Rev Drug Discov 19, 253–275 (2020).
Schauer C, Janko C, Munoz LE, Zhao Y, Kienhöfer D, Frey B, et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat Med 20, 511–517 (2014).
Rose DM, Sydlaske AD, Agha-Babakhani A, Johnson K, Terkeltaub R. Transglutaminase 2 limits murine peritoneal acute gout-like inflammation by regulating macrophage clearance of apoptotic neutrophils. Arthritis Rheum 54, 3363–3371 (2006).
Amaral FA, Costa VV, Tavares LD, Sachs D, Coelho FM, Fagundes CT, et al. NLRP3 inflammasome-mediated neutrophil recruitment and hypernociception depend on leukotriene B (4) in a murine model of gout. Arthritis Rheum 64, 474–484 (2012).
Oliviero F, Scanu A. How Factors Involved in the Resolution of Crystal-Induced Inflammation Target IL-1β. Front Pharmacol 8, 164 (2017).
Landis RC, Yagnik DR, Florey O, Philippidis P, Emons V, Mason JC, et al. Safe disposal of inflammatory monosodium urate monohydrate crystals by differentiated macrophages. Arthritis Rheum 46, 3026–3033 (2002).
Chen CJ, Shi Y, Hearn A, Fitzgerald K, Golenbock D, Reed G, et al. MyD88-dependent IL-1 receptor signaling is essential for gouty inflammation stimulated by monosodium urate crystals. J Clin Invest 116, 2262–2271 (2006).
Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 (2006).
Murakami Y, Akahoshi T, Hayashi I, Endo H, Kawai S, Inoue M, et al. Induction of triggering receptor expressed on myeloid cells 1 in murine resident peritoneal macrophages by monosodium urate monohydrate crystals. Arthritis Rheum 54, 455–462 (2006).
Martin WJ, Shaw O, Liu X, Steiger S, Harper JL. Monosodium urate monohydrate crystal-recruited noninflammatory monocytes differentiate into M1-like proinflammatory macrophages in a peritoneal murine model of gout. Arthritis Rheum 63, 1322–1332 (2011).
Locati M, Curtale G, Mantovani A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu Rev Pathol 15, 123–147 (2020).
Zhang B, Xu H, Chen J, Zhu X, Xue Y, Yang Y, et al. Highly specific and label-free histological identification of microcrystals in fresh human gout tissues with stimulated Raman scattering. Theranostics 11, 3074–3088 (2021).
Zhao L, Zhao T, Yang X, Cao L, Xu R, Liu J, et al. IL-37 blocks gouty inflammation by shaping macrophages into a non-inflammatory phagocytic phenotype. Rheumatology keac009 https://doi.org/10.1093/rheumatology/keac009 (2022).
Funding
This work was supported by National Natural Science Foundation of China (82071756) and the Research Funding from the Shanghai Hospital Development Center (SHDC2020CR1013).
Author information
Authors and Affiliations
Contributions
LZ carried out the mouse studies and cells studies and participated in the manuscript drafting. WJY performed the statistical analysis and participated in the manuscript drafting. YFZ and ZGD were responsible for the pathological assessment. FFC, XXL and QW performed the statistical analysis. YHH provided pathological specimens. XXZ and YYY helped to revise the manuscript. HJZ, LL and YX conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All procedures on animals followed guidelines established by the Institutional Animal Care Committee and the China Council on Animal Care at Fudan University (Shanghai, China). The collection of human samples was approved by Ethics Committee of Huashan Hospital. All human samples were obtained with the patient’s written informed consent which was approved by Ethics Committee of Huashan Hospital.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Zhao, L., Ye, W., Zhu, Y. et al. Distinct macrophage polarization in acute and chronic gout. Lab Invest 102, 1054–1063 (2022). https://doi.org/10.1038/s41374-022-00798-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41374-022-00798-4
This article is cited by
-
Comparison of the different monosodium urate crystals in the preparation process and pro-inflammation
Advances in Rheumatology (2023)
-
Upregulation of CD39 During Gout Attacks Promotes Spontaneous Remission of Acute Gouty Inflammation
Inflammation (2023)