Abstract
Endometrial carcinoma is one of the most common malignancies in the female reproductive system. Interleukin-37 (IL-37) is a newly discovered anti-inflammatory factor belonging to the IL-1 family. IL-37 has five different isoforms, and IL-37b is the most biologically functional subtype. In recent years, the protective roles of IL-37 in different cancers, including lung and liver cancers, have been successively reported. IL-37 also plays an important role in some gynecological diseases such as endometriosis, adenomyosis, and cervical cancer. However, the role and mechanism of IL-37b, especially the mature form of IL-37b, in endometrial carcinoma have not been elucidated. The present study demonstrated that IL-37 protein was downregulated in endometrial carcinoma cells compared with the control endometrium. IL-37b did not affect the proliferation and colony-forming ability of endometrial cancer cells. A mature form of IL-37b (IL-37bΔ1-45) effectively suppressed the migration and invasion of endometrial cancer cells by decreasing the expression of matrix metalloproteinase 2 (MMP2) via Rac1/NF-κB signal pathway. However, it did not affect epithelial–mesenchymal transition (EMT) or filamentous actin (F-actin) depolymerization of endometrial cancer cells. IL-37bΔ1-45 attenuated tumor metastasis in a peritoneal metastatic xenograft model of endometrial cancer. To sum up, these results suggested IL-37b could be involved in the pathogenesis of endometrial carcinoma and provide a novel target for the diagnosis and treatment of endometrial carcinoma.
Similar content being viewed by others
Introduction
Endometrial carcinoma is one of the most common malignancies in the female reproductive system [1, 2]. The vast majority (>90%) of endometrial carcinomas are found in women aged over 50 years; only very few women suffered under the age of 35 [3]. According to pathogenesis and biological behavior characteristics, endometrial carcinomas can be divided into two types (Type I and Type II) [4, 5]. Among them, more than 80% of endometrial carcinomas are endometrioid adenocarcinomas, which are estrogen-dependent (type I endometrial carcinomas) [1, 6,7,8,9]. Type II endometrial carcinomas are estrogen-independent, such as uterine papillary serous carcinoma and clear cell carcinomas [7]. Nowadays, the etiology of endometrial carcinoma is unclear, and its risk factors may be relevant to fat, diabetes, hypertension, and menstrual disorder, etc. With the development of modern treatment strategies, the survival rates of patients with endometrial carcinoma have significant improvements. However, many patients are not sensitive to traditional treatment methods and easy to be recurrence and metastasis. Therefore, understanding the underlying mechanism of tumor cell proliferation, migration, and invasion will be of great value for exploring new targets to treat endometrial carcinoma patients.
Interleukin-37 (IL-37) is a novel anti-inflammatory cytokine discovered by bioinformatics analysis in 2000 [10]. IL-37 is one of the IL-1 family member, which includes pro-inflammatory factors (IL-1α and IL-1β, IL-18, IL-33, IL-36α, IL-36β, IL-36γ) and anti-inflammatory factors (IL-37 and IL-38). IL-37 plays a critical inhibitory role in innate and adaptive immunity by directly decreasing pro-inflammatory cytokines’ production. The gene encoding IL-37 is located on human chromosome 2 [11, 12]. Interestingly, there is no mouse-derived IL-37 gene so far [13, 14]. The human IL-37 gene needs to undergo a process of variable splicing [15], thus forming different subtypes: IL-37a (21.55kD), IL-37b (24.13kD), IL-37c (19.61kD), IL-37d (21.95kD), IL-37e (17.46kD). Among them, IL-37b is the biggest subtype and contains exons 1, 2, 4, 5, and 6. Therefore, it may be the most biologically functional subtype. Two mature forms of IL-37b, D20-218 (IL-37bΔ1-20), and V46-218 (IL-37bΔ1-45), are generated through protease cleavages. Mature D20-218 is cleaved in the cytoplasm by activated caspase-1 at residue 20 (Asp). Another mature form, V46-218 is possibly cleaved by neutrophil secreted protease in the supernatants of IL-37 gene-transfected human embryonic kidney 293 cells. This form shows higher biologic activity than the longer (amino acids 20–218) [16]. IL-37 expresses in a variety of tissues and organs and has evident tissue specificity [17]. It can be found in the cytoplasm or nucleus of cells, and it is also secreted [18]. In recent years, the protective roles of IL-37 in different cancers [19], including lung and liver cancer, have been successively reported [20, 21]. IL-37 also plays a vital role in some gynecological diseases such as endometriosis [22,23,24,25,26], adenomyosis [27], and cervical cancer [28]. However, the role and mechanism of IL-37b, especially the mature form of IL-37b, in endometrial carcinoma have not been reported.
In the present study, we detected the expression of IL-37 in the endometrial carcinoma tissues and explored the effect and molecular mechanism of IL-37b overexpression or knockdown on proliferation, migration, and invasion of endometrial cancer cells. The impact of IL-37b on metastasis of endometrial cancer cells in nude mice was also studied. The results demonstrated that IL-37 protein expression was decreased in endometrial carcinoma cells compared with the control endometrium. A mature form of IL-37b (IL-37bΔ1-45) inhibited the migration, invasion, and metastasis of endometrial cancer cells in vitro and in vivo, and the mechanism was related to Rac1/NF-κB/MMP2 signal pathway, suggesting IL-37b was a new target for treating endometrial carcinoma.
Materials and methods
Sample collection
Forty paraffin-embedded specimens and sixteen freshly frozen (stored in the −80 °C) specimens were obtained from endometrioid adenocarcinoma patients aged 44–74 years who underwent primary surgeries in the Department of Gynecology, Jinan Central Hospital, Shandong University. The patients had not received any hormone therapy, radiotherapy, or chemotherapy before surgery. The clinical-stage was assessed according to the International Federation of Gynecology and Obstetrics (FIGO) system (2009) [29]. Tumor differentiation degree, depth of myometrial invasion (MI), the expression of estrogen receptor (ER), and progesterone receptor (PR) were also evaluated. The essential characteristics of endometrioid adenocarcinoma patients were listed in Table 1. Forty-six paraffin-embedded control endometrial specimens were obtained from surgical patients with non-endometrial diseases and divided into proliferative (n = 26) or secretory phases (n = 20) according to the patients’ menstrual history and histopathological examination [30], and 16 fresh control endometrial specimens were collected and frozen in the −80 °C. The Institutional Ethics Committee of Shandong University approved this study and the collection of all human samples, and all of the patients gave their informed consent.
Cell culture
Human endometrial cancer cell line (Ishikawa, ISK) was kindly gifted by Qilu Hospital, Shandong University, and maintained in high glucose DMEM (Hyclone, Logan City, Utah, USA) supplemented with 10% fetal bovine serum (Gibco Carlsbad, CA, USA). Human endometrial cancer cell lines (HEC-1-A, AN3CA, and RL95-2) were purchased from the China Center for Type Culture Collection (Wuhan, Hubei, China). HEC-1-A cells were grown in McCoy’s 5A medium (Gibco, Carlsbad, CA, USA) with 10% fetal bovine serum (Gibco, Carlsbad, CA, USA). AN3CA cells were cultured in MEM containing 1% non-essential amino acid and 1% sodium pyruvate supplemented with 10% fetal bovine serum (Gibco, Carlsbad, CA, USA). RL95-2 cells were cultured in DMEM/F12 supplemented with 10% fetal bovine serum (Gibco, Carlsbad, CA, USA). All of these cell lines were routinely cultured at 37 °C in a humidified atmosphere with 5% CO2.
Hormones treatment
Ishikawa and HEC-1-A cells were respectively treated with 0, 0.1, 1, 10, 100, 1000 nM of 17β-estradiol (E2, E2758-250MG, Sigma-Aldrich, St Louis, MO, USA) or 0, 0.01, 0.1, 1, 10, 20 μM of progesterone (P4, P0130-25G, Sigma-Aldrich, St Louis, MO, USA). The protein from all treated cells was collected to detect the expression of IL-37 using western blot.
Antibodies, Plasmids, siRNA, and transient transfection
The primary antibody against IL-37 (PA5-28817) was from Thermo Scientific (Waltham, MA, USA). The primary antibody against IL-37 (60296-1-Ig) was from proteintech (Wuhan, Hubei, China). Primary antibodies against MMP2 (ab92536), MMP9 (ab76003) and Rac1 (ab33186) were from Abcam (Cambridge, UK). Primary antibodies against Flag (F1804-50UG), Myc (M4439-100UL) were from Sigma- Aldrich (St Louis, MO, USA). Primary antibodies against E-Cadherin (3195), N- Cadherin (13116), Vimentin (5741), p-PAK1/2/3 (2604), p-Iκκα/β (2697), p-IκBα (9246), p-AKT (4060), AKT (4691), p-P65 (3033), P65 (8242), p-SAPK/JNK (4668), SAPK/JNK (9252), p-p44/42MAPK (ERK1/2) (4370), p44/42MAPK (ERK1/2) (4695), p-p38MAPK (4511), p38MAPK (8690) were from Cell Signaling Technology (Danvers, MA, USA). The primary antibody against β-actin was from ZSGB-Bio (Beijing, China).
All plasmid vectors for pcDNA3.1/HisC/IL-37 (Full-length human IL-37b, IL-37bΔ1-45, and IL-37bΔ1-20) with a C-terminal 3×flag and PRK5/Rac1 (Rac1- 61L) with an N-terminal Myc were kindly gifted by the Team of Zhang Lining in Department of Immunology, School of Basic Medical Sciences, Shandong University. Specific siRNAs for IL-37 were as follows: siIL-37-1: 5′-GCAUUAGCCUCAU CCUUGAUU-3′ and 5′-UCAAGGAUGAGGCUAAUGCUU-3′; siIL-37-2: 5′-GAG AACAGGAAACACAUUGUU-3′ and 5′-CAAUGUGUUUCCUGUUCUCUU-3′; siIL-37-3: 5′-UCUACUGUGACAAGGAUAAUU-3′ and 5′-UUAUCCUUGUCAC AGUAGAUU-3′; negative control (NC): 5′-UUCUCCGAACGUGUCACGUTT-3′ and 5′-ACGUGACACGUUCGGAGAATT-3′. Transfection with plasmids was performed using Lipofectamine2000 (Invitrogen, Carlsbad, CA, USA). Transfection with siRNAs was performed using INTERFERin (PolyPlus, Strasbourg, France).
Immunohistochemistry (IHC)
The specimens were embedded in paraffin and sectioned (5 μm) by Servicebio (Wuhan, Hubei, China). Tissue sections were deparaffinized in xylene and rehydrated in graded ethanol routinely. Antigen retrieval was performed with a high-pressure method. Endogenous peroxidase was blocked with 3% H2O2 for 10 min at room temperature, and nonspecific binding was blocked with 10% goat serum for 15 min at 37 °C. Rabbit polyclonal antibody against IL-37 (PA5-28817, 1:300, Thermo Scientific) was added to the slides separately, and the slides were incubated overnight at 4 °C in a wet chamber. The next day, the slides were balanced at room temperature for 30 min and incubated with HRP-conjugated goat anti-rabbit IgG for 1 h at 37 °C. The sections were then stained with 3,3′-diaminobenzidine (DAB, 1:20, ZSGB-Bio, Beijing, China). Finally, all sections were counterstained with hematoxylin, differentiated with 1% acid alcohol, backed to blue with 1% ammonium, and covered with glasses.
The intensity and extent of positive staining were evaluated by the observers blinded to the clinical information. The sum of staining intensity and positive area percentage was used as the final score of IL-37. A total score of 0 indicated no expression; a total score of 1 and 2 indicated weak expression; a total score of 3 and 4 indicated moderate expression; a total score of 5 and 6 indicated strong expression.
Quantitative real-time PCR (qPCR)
Total RNA was extracted using Trizol Reagent (TIANGEN, Beijing, China) and reversely transcribed into cDNA with Reverse Transcription System (Takara, Shiga, Japan). QPCR was performed on the LightCycler®96 system (Roche, Basle, Switzerland) using UltraSYBR Mixture (CWBIO, Beijing, China). The levels of gene expression were normalized to GAPDH and analyzed by the 2−ΔΔCt method. Each sample was examined in triplicate. The primer sequences were listed in Table 2.
Western blot
The samples were lysed using RIPA lysis buffer (Beyotime, Beijing, China) containing protease and phosphatase inhibitors (Bimake, Houston, TX, USA). After centrifugation, the supernatant was quantified using a BCA assay kit (Thermo Scientific, Waltham, MA, USA). An equal amount of protein was separated using sodium dodecyl sulfate-polyacrylamide gel and then transferred onto the polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). The membranes were blocked with (TBST) containing 5% bovine serum albumin (Sigma-Aldrich, St Louis, MO, USA) for 2–3 h, and then respectively incubated with primary antibodies overnight at 4 °C. The next day, the membranes were incubated with HRP-conjugated secondary antibodies (1:5000, Jackson Immuno Research, West Grove, PA, USA) at room temperature for 1 h. The signal was detected by the enhanced chemiluminescence kit (Millipore, Billerica, MA, USA).
Cell counting Kit-8 (CCK-8) assay
IL-37 overexpressing/silenced cells were seeded at a density of 1 × 104 Ishikawa cells or 2 × 104 AN3CA cells per well in 96-well plates. Cell viability was detected using CCK-8 (Dojindo Laboratories, Japan) at the indicated time points (0, 24, 48, and 72 h). The absorbance was determined at 450 nm wavelength. Triplicate was performed in each experimental group.
Colony formation assay
IL-37 overexpressing/silenced cells were seeded at a density of 1000 Ishikawa cells or 3000 AN3CA cells per well in 6-well plates and cultured at 37 °C for 10 or 18 days, changing the medium every 3 days. At the end of the incubation, the cells were fixed with methanol and stained with crystal violet (Beyotime, Beijing, China). The number of colonies containing more than 50 cells was counted. Each sample was performed in triplicate.
Transwell migration and invasion assay
For migration assay, IL-37 overexpressing/silenced cells were suspended in the medium with 0.1% FBS. After counted, the same number of cells (150 μl) were added into the upper chambers with 8 μm pore size (Greiner bio-one, Frickenhausen, Germany), and the medium containing 20% FBS (650 μl) was added into the lower chamber. For invasion assay, the upper chamber was covered with Matrigel (Corning Incorporated, New York, USA) in advance. After incubation for 24 or 48 h, the cells were fixed with methanol and then stained with crystal violet. The cells on the upper side were wiped off, and the cells that adherent to the underside of the membrane were remained. Five randomly selected fields were counted under a light microscope at ×100 magnifications using an Olympus DP72 digital camera and DP Controller software (Olympus, Tokyo, Japan). Each experiment was performed in triplicate.
Immunofluorescence
IL-37bΔ1-45 overexpressing cells were seeded in 24-well plates with coverslips for 24 h at 37 °C. The cells on coverslips were fixed for 10 min with 4% paraformaldehyde, permeabilized for 10 min with 1% Triton X-100 (T8200, solarbio), and blocked for 1 h with 3% BSA. The cells were then incubated with the phalloidin- Tetramethylrhodamine B isothiocyanate (TRITC-conjugated phalloidin) (P1951-1MG, Sigma-Aldrich, St Louis, MO, USA) at 37 °C for 1 h in a dark place. The nuclei were stained for 5 min with 4′,6-diamidino-2-phenylindole (C1005, Beyotime). Images were observed using a VS120 (Olympus, Tokyo, Japan).
PAK-PBD pull-down assay
Flag-IL-37bΔ1-45 plasmid transfected Ishikawa cells were incubated at 37 °C for 24 h. The protein was collected using Rac1 Activation Assay Biochem Kit (20 Rxns, BK035-S, Cytoskeleton, Denver, CO, USA). An equal amount of cell lysate was incubated with 20 μg of PAK-PBD protein beads for 1 h at 4 °C with rotation. Active GTP-bound Rac1 could be pulled down by PAK-PBD protein beads. The pull-down fractions and total protein were analyzed by western blot.
Co-IP assay
Flag-IL-37bΔ1-45 and Myc-Rac1-61L plasmids were co-transfected in Ishikawa cells at 37 °C for 24 h. The protein was extracted with 500 μl IP buffer. After centrifuged, 400 μl of prepared cell lysate (IP group) was incubated with 1.5 μl mouse-derived monoclonal antibody (anti-Flag) for 1 h at 4 °C with rotation. After that, 40 μl re-suspended Protein A/G Plus-Agarose (Sc-2003, Santa Cruz, Dallas, TX, USA) was added and incubated for 12–16 h at 4 °C with rotation. The bound fractions were washed three times with 500 μl IP buffer, then 40 μl of 2× Laemmli buffer was added. Eight microliters of 6× Laemmli buffer were added to 40 μl of prepared cell lysate (Input group). The proteins in the IP group and the Input group were boiled for 5 min in a metal bath. The IP group and input group were analyzed by western blot.
Endometrial cancer peritoneal metastatic xenograft model
CV146/luciferase/puro lentiviral vectors with IL-37bΔ1-45 or NC were constructed by Genechem Company (Shanghai, China). Lentivirus infection was performed according to the manufacturer’s protocol (Ishikawa cell line, MOI = 20; 25× HiTransGA). After infection, puromycin (3 μg/ml) was used for screening, qPCR and western blot were performed for identification. The stably expressed cells (ISK-LV-IL-37bΔ1-45 and ISK-LV-NC) were established.
Four-week-old female BALB/c nude mice were purchased from Beijing Vital River Laboratories (Beijing, China) and housed under Specific pathogen Free conditions. The mice were randomly divided into two groups (n = 10/each group). The peritoneal metastatic xenograft models of endometrial cancer were established by intraperitoneal injection of ISK-LV-IL-37bΔ1-45 cells or ISK-LV-NC cells (2 × 106 in 200 μl of normal saline/each mouse). Thirty-eight days later, all mice were injected intraperitoneally with a substrate D-luciferin (abs42017256, Absin Bioscience, Shanghai, China). Ten minutes later, the mice were injected intraperitoneally with 0.3% pentobarbital sodium solution. Five minutes later, in vivo bioluminescence imaging was performed in the above mice using the In Vivo Imaging System spectrum (PerkinElmer, Santa Clara, CA, USA). Then the mice were killed, and the organs (liver, lung, and kidney) were removed out for imaging. After that, the part of tumors and the above organs were fixed in formalin and embedded in paraffin, and the rest was frozen at −80 °C for HE staining, IHC staining, and western blot. All animal care and experiments were approved by the Animal Ethics Committee of Shandong University and accorded with the Guidelines for the Care and Use of Laboratory Animals of Shandong University (Jinan, Shandong, China).
Statistics analysis
All statistical analyses were performed using GraphPad Prism 7.0 software (La Jolla, CA, USA). All data were presented as means ± standard deviations. A two-tailed unpaired student’s t-test was performed to evaluate the test and control groups’ statistical significance. The Chi-square test was used to analyze the results from IHC. After a one way ANOVA, a Dunnett’s post-hoc test was used to compare different test groups with one control group. P < 0.05 was considered a statistically significant difference.
Results
The expression of IL-37 mRNA and protein was upregulated in endometrioid adenocarcinoma tissues detected by qPCR and western blot
To investigate the expression status of IL-37 in endometrioid adenocarcinomas, we firstly detected the IL-37 mRNA and protein expression in control endometrium and endometrioid adenocarcinoma tissues using qPCR and western blot. We found that IL-37 mRNA and protein levels were upregulated in endometrioid adenocarcinoma tissues compared with control endometrium (p < 0.01, Supplementary Fig. 1A, B).
The expression of IL-37 protein was downregulated in endometrioid adenocarcinoma cells detected by IHC
To explore the expression sites and levels of IL-37 protein, we detected the expression of IL-37 in endometrioid adenocarcinoma tissues and control endometrium using IHC. The results showed that IL-37 positive staining was localized in the cytoplasm of glandular epithelial cells, while no evident positive staining could be observed in endometrial stromal cells (Fig. 1A). Statistical analysis results confirmed that the levels of IL-37 protein were significantly reduced in endometrioid adenocarcinoma cells compared with the control endometrium (p < 0.0001, Fig. 1B), which suggested that the occurrence of endometrioid adenocarcinomas might have a relationship with IL-37.
A Representative IHC staining for IL-37 protein in endometrioid adenocarcinoma tissues and control endometrium (Scale bar, 100μm). (a) The proliferative phase of control endometrium; (b) The secretory phase of control endometrium; (c) The well differentiation of endometrioid adenocarcinoma; (d) The moderate differentiation of endometrioid adenocarcinoma; (e) The poor differentiation of endometrioid adenocarcinoma. B Statistical analysis of IL-37 protein expression detected by IHC showed that the levels of IL-37 protein were significantly reduced in endometrioid adenocarcinoma cells compared with the control endometrium (p < 0.0001). C In the control endometrium, the expression of IL-37 protein in the proliferative phase was significantly reduced compared with that in the secretory phase (p < 0.01). Progesterone (D) and 17β-estradiol (E) did not affect the expression of IL-37 protein in Ishikawa and HEC-1-A cells detected by western blot. P4, progesterone; E2, 17β-estradiol. **p < 0.01; ****p < 0.0001.
Among 40 cases of endometrial carcinoma tissues, we further analyzed the relationship between IL-37 expression levels and clinicopathological parameters. Table 1 showed no significant correlations between IL-37 and tumor differentiation degree, FIGO stage (p > 0.05). However, we found that the expression of IL-37 was significantly related to age, myometrial invasion, ER, or PR (p < 0.05).
Estrogen and progesterone did not affect the expression of IL-37 protein in endometrial cancer cells
It has been reported that the expression of IL-37 could be regulated by corticosteroids [31, 32]. To explore whether the expression of IL-37 protein could be affected by ovarian steroid hormones, we compared the differences in IL-37 expression between the proliferative and secretory phases of the control endometrium. It was found that the expression of IL-37 protein in the proliferative phase (n = 26) was significantly reduced compared with that in the secretory phase (n = 20) of the control endometrium (p < 0.01, Fig. 1C). However, Ishikawa and HEC-1-A cells were treated with different estrogen or progesterone concentrations, and the results showed that the expression of IL-37 was not regulated by estrogen and progesterone at protein levels (Fig. 1D, E).
IL-37b overexpression or knockdown increased or decreased the expression of IL-37 at mRNA and protein levels
To choose the suitable cell lines, we detected the expression of IL-37 in different endometrial cancer cell lines at mRNA and protein levels. As shown in Fig. 2A, B, Ishikawa cells had a relatively low IL-37 expression, while AN3CA cells had a relatively high expression. Therefore, the overexpression efficiency was detected after transfecting IL-37bΔ1-45, IL37bΔ1-20, and IL-37b full-length plasmids in Ishikawa cells. The identification of IL-37 inference efficiency was tested after transfecting three specific siRNAs targeting IL-37 in AN3CA cells. The results showed that IL-37bΔ1-45, IL37bΔ1-20, and IL-37b were expressed at mRNA and protein levels in Ishikawa cells (Fig. 2C). Moreover, siIL-37-1 and siIL-37-3 could effectively decrease the expression of IL-37 mRNA and protein (p < 0.05, Fig. 2D, E).
The relative expression of IL-37 mRNA (A) and IL-37 protein (B) in human endometrial cancer cells (Ishikawa, HEC-1-A, RL95-2, AN3CA). The human hepatocellular carcinoma cell (Bel-7402) was used as a positive control. C Ishikawa cells transfected with IL-37 overexpression plasmids (IL-37bΔ1-45, IL-37bΔ1-20, and IL-37b full-length) showed higher expression of IL-37 than MOCK group detected by qPCR and western blot. D AN3CA cells transfected with siIL-37-1, siIL-37-2, and siIL-37-3 showed lower expression of IL-37 mRNA than NC group detected by qPCR (p < 0.05). E AN3CA cells transfected with siIL-37-1 and siIL-37-3 showed lower expression of IL-37 protein than NC group detected by western blot (p < 0.01). NC negative control, ns no significant. *p < 0.05; **p < 0.01; ***p < 0.001.
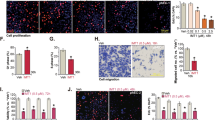
IL-37b did not affect the proliferation and colony formation ability of endometrial cancer cells
IL-37 can inhibit the proliferation of human cervical cancer cells [28] and hepatocellular carcinoma cells [21]. Here, we examined the effects of IL-37b on proliferation and colony formation ability after transfecting respectively MOCK, IL-37bΔ1-45, IL-37bΔ1-20, and IL-37b full-length plasmids in Ishikawa cells. We also detected the effects of IL-37 specific siRNAs on proliferation and colony formation ability in AN3CA cells. The results from CCK-8 and colony formation assay showed that IL-37b had no impact on the proliferation and colony formation ability of endometrial cancer cells (p > 0.05, Fig. 3A–D). Cell proliferation is related to the activation of MAPK and PI3K/AKT signal pathways. To further confirm the effect of IL-37b on cell proliferation in endometrial cancer cells, we examined the expression of proliferation-related signal molecules (p-ERK1/2, p-P38, p-JNK1/2, and p-AKT) after transfecting respectively MOCK, IL-37bΔ1-45, IL-37bΔ1-20, and IL-37b full-length plasmids in Ishikawa cells. The results showed that these molecules had no visible changes between the test group and the control group (Fig. 3E).
IL-37b overexpression in Ishikawa cells (A) and IL-37 knockdown in AN3CA cells (B) did not affect the proliferation of endometrial cancer cells detected by CCK8 assay (p > 0.05). IL-37b overexpression in Ishikawa cells (C) and IL-37 knockdown in AN3CA cells (D) did not affect the colony-forming ability of endometrial cancer cells detected by the colony formation assay (p > 0.05). E The expression of p-ERK1/2, p-P38, p-JNK1/2, and p-AKT had no obvious changes after transfecting respectively MOCK, IL-37bΔ1-45, IL-37bΔ1-20, and IL-37b full-length plasmids.
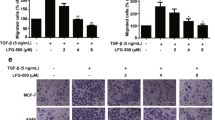
IL-37b suppressed the migration and invasion ability of endometrial cancer cells
Migration and invasion of endometrial cells are the main characteristics of endometrial cancer [33]. Previous reports have shown that IL-37 plays an inhibitory role in the migration and invasion of some cancer cells, such as A549 cells [20] and SMMC-7721 cells [21]. To determine the roles of IL-37 in the migration and invasion of endometrial cancer cells, we detected the effect of IL-37b overexpression on the migration or invasion ability in Ishikawa cells using transwell migration assay or matrigel invasion assay. The results showed that IL-37bΔ1-45 overexpression in Ishikawa cells resulted in a significant reduction in the number of cells passing through the chambers than the MOCK group (p < 0.05, Fig. 4A, B, Supplementary Fig. 2A, B). We also detected the effect of IL-37 knockdown on the migration and invasion ability in AN3CA cells using transwell migration assay and matrigel invasion assay. As we expected, IL-37 knockdown in AN3CA cells increased the number of cells migrating or invading the transwell membrane’ lower surface (p < 0.05, Fig. 4C, D). These data indicated that IL-37bΔ1-45 could suppress the migration and invasion ability of endometrial cells.
IL-37bΔ1-45 overexpression in Ishikawa cells resulted in a significant reduction in the number of cells migrating (A) or invading (B) the lower surface of chambers compared with the MOCK group (p < 0.05). IL-37 knockdown in AN3CA cells resulted in a significant increase in the number of cells migrating (C) and invading (D) the lower surface of the chamber compared with the NC group (p < 0.05). *p < 0.05; **p < 0.01; ****p < 0.0001.
IL-37b had no clear impact on epithelial–mesenchymal transition (EMT) or filamentous actin (F-actin) depolymerization of endometrial cancer cells
It has been reported that IL-37 suppresses the migration and invasion of gallbladder cancer cells through the inhibition of HIF-1α induced EMT [34]. To examine IL-37 and EMT’s relationship, we examined the markers of epithelial cells (E-cadherin) and stromal cells markers (N-cadherin, Vimentin) and Twist at mRNA and protein levels. The results showed that IL-37bΔ1-45 overexpression did not affect EMT (p > 0.05, Supplementary Fig. 3A, B). Cell migration is critical to cancer cell invasion and metastasis and relates to cytoskeletal remodeling [35, 36]. We used immunofluorescence to examine the effect of IL-37Δ1-45 overexpression on F-actin in AN3CA cells. We found that F-actin depolymerization was not significantly changed in IL-37bΔ1-45 overexpressed group compared with the MOCK group (p > 0.05, Supplementary Fig. 3C). PAK (p21-activated kinases) enables actin filaments to continue growing [37]. We further tested the expression of p-PAK using western blot after transfecting IL-37bΔ1-45 and found that the expression of p-PAK in Ishikawa cells and AN3CA cells had no significant differences between IL-37bΔ1-45 overexpressed group and MOCK group (Supplementary Fig. 3D).
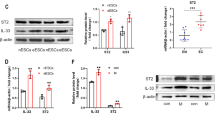
IL-37b decreased the expression of MMP2 by Rac1/NF-κB signal pathway in endometrial cancer cells
Extracellular matrix (ECM) degradation contributes to cancer cell invasion [38]. MMP2 and MMP9 are the key molecules that degrade the basement membrane [39]. Therefore, qPCR and western blot were performed to detect the effect of IL-37 on the expression of MMP2 and MMP9 in endometrial cancer cells. The qPCR results showed that IL-37b overexpression in Ishikawa cells did not affect MMP2 and MMP9 mRNA (p > 0.05, Fig. 5A). However, western blot results showed that IL-37b overexpression in Ishikawa cells resulted in a significant reduction of MMP2 protein (p < 0.05), the expression of MMP9 protein had no obvious change (p > 0.05, Fig. 5B). Inversely, IL-37 knockdown in AN3CA cells increased the expression of MMP2 protein (p < 0.05) but did not affect the level of MMP9 protein (p > 0.05, Fig. 5C).
A IL-37b overexpression in Ishikawa cells did not affect the MMP2 and MMP9 mRNA levels detected by qPCR (p > 0.05). B IL-37b overexpression in Ishikawa cells downregulated the levels of MMP2 protein detected by western blot (p < 0.05). However, the expression of MMP9 protein had no significant change (p > 0.05). C IL-37 knockdown in AN3CA cells increased the expression of MMP2 protein detected by western blot (p < 0.05). However, the expression of MMP9 protein had no significant change (p > 0.05). D IL-37b overexpression decreased the expression of p-P65 detected by western blot (p < 0.05), but the expression of p-Iκκα/β and p-IκBα had no significant change. E IL-37 knockdown increased the expression of p-P65 detected by western blot (p < 0.05). F IL-37bΔ1-45 overexpression in Ishikawa cells inhibited Rac1 activation detected by western blot. G IL-37bΔ1-45 binds to Rac1-61L after Myc-tagged Rac1-61L and 3×Flag-tagged IL-37bΔ1-45 plasmids were co-transfected into Ishikawa cells. The immunoprecipitation was performed with anti-Flag antibody, and the precipitates and lysates were analyzed by immunoblotting with antibodies for Myc and Flag. *p < 0.05; **p < 0.01.
The NF-κB signal pathway is closely related to the expression of MMP2. Previous reports showed that IL-37 could participate in the above signal pathway [40, 41]. To further explore the mechanism of IL-37b affecting the expression of MMP2 protein, we detected the expression of relative molecules (p-P65, p-IκBα, p-Iκκα/β) in the NF-κB signal pathway after transfecting IL-37b plasmids in Ishikawa cells, and we found that IL-37b only reduced the expression of p-P65 (p < 0.05, Fig. 5D). On the contrary, IL-37 silence could upregulate the expression of p-P65 (p < 0.05, Fig. 5E).
It has been reported that intracellular mature IL-37 suppresses tumor metastasis via inhibiting Rac1 activation [42]. Here, we examined the effect of IL-37bΔ1-45 on the activation of Rac1 using PAK-PBD pull-down assay and found that IL-37bΔ1-45 overexpression inhibited the expression of GTP-Rac1 in Ishikawa cells (Fig. 5F). Furthermore, the interaction between IL-37bΔ1-45 and Rac1-61L was confirmed using Co-IP after co-transfecting Flag-IL-37bΔ1-45 and Myc-Rac1-61L plasmids in Ishikawa cells. The result showed that IL-37bΔ1-45 could bind to Rac1-61L (Fig. 5G).
IL-37b suppressed the migration and invasion ability of endometrial cancer cells by the Rac1/NF-κB/MMP2 signal pathway
IL-37bΔ1-45 is the most efficient in inhibiting the migration and invasion of endometrial cancer cells. Therefore, we constructed IL-37bΔ1-45-stable expressed cell line and control cell line by infecting IL-37Δ1-45 lentivirus or NC lentivirus into Ishikawa cells. The qPCR and western blot results confirmed that IL-37bΔ1-45-stable expressed cell line and control cell line were successfully constructed, and stable IL-37bΔ1-45 expression reduced the levels of MMP2 protein (Fig. 6A). Moreover, the migration and invasion ability of IL-37bΔ1-45-stable expressed cell line were decreased compared with the control cell line (p < 0.05, Fig. 6B, C).
A Ishikawa cells infected with IL-37bΔ1-45 lentivirus (LV-IL-37bΔ1-45) showed higher expression of IL-37 than that infected with negative control lentivirus (LV-NC) detected by qPCR and western blot (p < 0.0001). The expression of MMP2 protein was downregulated in Ishikawa cells with IL-37bΔ1-45 lentivirus detected by western blot. LV-IL-37bΔ1-45 decreased significantly the number of cells migrating (B) or invading (C) the lower surface of chambers compared with the LV-NC group (p < 0.05). D The inhibitory effect of IL-37bΔ1-45 on MMP2 and p-P65 was reversed after the Rac1-61L overexpression plasmid was used to transfect LV-IL-37bΔ1-45 stably expressed cells (ISK-LV-IL-37bΔ1-45). Rac1-61L overexpression significantly increased the number of IL-37bΔ1-45 stably expressed cells migrating (E) or invading (F) the lower surface of chambers compared with the MOCK group (PRK5 control plasmid) (p < 0.05). LV Lentivirus. *p < 0.05; **p < 0.01; ***p < 0.001.
Besides, Rac1/NF-κB/MMP2 signal pathway was further identified by transfecting Myc-Rac1-61L plasmid in IL-37bΔ1-45-stable expressed cell line. The result showed that the expression of p-P65 and MMP2 were upregulated after transfecting Rac1-61L (Fig. 6D), and the migration and invasion ability of IL-37bΔ1-45-stable expressed cell line were also increased (p < 0.05, Fig. 6E, F).
IL-37b attenuated tumor metastasis in a peritoneal metastatic xenograft model of endometrial cancer
To provide in vivo evidence that IL-37 suppressed endometrial cancer cells’ metastasis, we established a peritoneal metastatic xenograft model of endometrial cancer in female BALB/c nude mice using an intraperitoneal injection of IL-37b Δ1-45-stable expressed endometrial cancer Ishikawa cells and the parental endometrial cancer Ishikawa cells. Thirty-eight days later, the results from an in vivo imaging technique showed that the LV-IL-37bΔ1-45 group had a significantly reduced tumor metastasis compared with the LV-NC group (Fig. 7A, B). Image results of liver, kidney, and lung confirmed that IL-37bΔ1-45 could substantially decrease liver metastasis (Fig. 7C). However, the weight of the mice was monitored every week, and the results showed no significant differences between the two groups (Fig. 8A). The results from an anatomic diagram in the abdomen and HE staining further confirmed that the peritoneal metastatic xenograft model of endometrial cancer had been successfully established (Fig. 8B, C). The results of IHC showed that IL-37 levels in the LV-IL-37bΔ1-45 group were higher than those in the LV-NC group (Fig. 8D). Western blot results confirmed that the expression of MMP2 was downregulated in the LV-IL-37bΔ1-45 group compared with the LV-NC group (Fig. 8E).
A, B In vivo imaging technique showed that the LV-IL-37bΔ1-45 group had a significantly reduced tumor metastasis compared with the LV-NC group. The bioluminescence was quantified by determining the total flux (photons/sec; p/s) in each ROI. Data acquired from supine (A) and prone (B) views were shown. Data were represented as mean ± SD. n = 10 for each group (p < 0.05). C Image results of liver, kidney, and lung confirmed that IL-37bΔ1-45 could significantly decrease liver metastasis. The bioluminescence quantification represented that IL-37bΔ1-45 inhibited liver metastasis (p < 0.05). *p < 0.05; **p < 0.01.
A The weight curves of female BALB/c nude mice were shown after intraperitoneal injection with the stably expressed cells (ISK-LV-NC and ISK-LV-IL-37bΔ1-45), and the IL-37bΔ1-45 had no relationship with mice weight (p > 0.05). B The anatomical images of female BALB/c nude mice were represented after intraperitoneal injection with the stably expressed cells (ISK-LV-NC and ISK-LV-IL-37bΔ1-45). C HE staining results confirmed the peritoneal metastatic xenograft model of endometrial cancer had been successfully established (Scale, 100 μm). D IL-37 levels in the LV-IL-37bΔ1-45 group were higher than those in the LV-NC group detected by IHC. E The expression of MMP2 was downregulated in the LV-IL-37bΔ1-45 group compared with the LV-NC group detected by western blot. *p < 0.05; **p < 0.01.
Discussion
IL-37 is a newly discovered immune negative regulator. It has anti-inflammatory properties in immune responses through the downregulation of pro-inflammatory molecules [43] and plays an important role in tumorigenesis [19]. The present study is the first illustration of the functions of IL-37 in endometrial carcinoma. Our results confirmed the metastasis of endometrial carcinoma was inhibited by heightening the expression of IL-37 in vitro and in vivo. Understanding mechanisms by which IL-37 inhibits endometrial carcinoma’s metastasis is likely to reveal specific and effective targets for endometrial carcinoma treatment.
In the present study, qPCR and western blot results showed that IL-37 mRNA and protein were upregulated in endometrioid adenocarcinoma tissues compared with control endometrium. However, IHC results confirmed that the levels of IL-37 protein were significantly lower in endometrioid adenocarcinoma cells than those in the control endometrium. Osborne et al. [44] reported that IL-37 mRNA was upregulated in lymphocytes (T, B, and natural killer cells) of the melanoma patients’ blood, suggesting IL-37 could be highly expressed in inflammatory cells. The inconsistency between the results of qPCR, western blot, and IHC may be the reason that qPCR and western blot could not exclude the expression of IL-37 in inflammatory cells. The results from IHC were meaningful because IHC could be used to determine the expression of IL-37 protein in endometrioid adenocarcinoma cells. The decreased expression of IL-37 protein in endometrioid adenocarcinoma cells suggested IL-37 could play inhibitory roles in endometrial cancer. Besides, the relationship between decreased IL-37 protein expression and clinicopathological parameters showed that the expression of IL-37 was significantly related to age, myometrial invasion, estrogen, or progestin receptor. Yan et al. [45] reported that the downregulation of IL-37 was significantly correlated with cancer stage, nodal involvement, invasion depth, distant metastasis, differentiation, and it was also shown to be an independent prognostic indicator for patients with colon cancer. The true clinical significance of IL-37 in endometrial cancer needs further exploration.
Endometrial cancer, one of the most common gynecologic malignancies, is a hormonally regulated disease [46]. Here, we found that the expression of IL-37 was different between the proliferative and secretory phases of the control endometrium. The previous study reports that progesterone and estradiol exert an inhibitory effect on anti-inflammatory cytokine (IL-10) production [47]. Like IL-10, IL-37 is also a kind of anti-inflammatory cytokine [48]. It is identified that IL-37 has some relationship with endocrine hormones [31, 32, 49]. The above results suggest that the expression of IL-37 may be regulated by ovarian hormones (estradiol and progesterone) in endometrial cancer cells. However, our results suggested that IL-37 was not regulated by estradiol and progesterone. There may be other reasons why there are differences in IL-37 expression between the control endometrium’s proliferative and secretory phases.
IL-37 has five subtypes; among them, IL-37b may be the most biologically functional subtype [16]. In the study, we found that the mature form of IL-37b (IL-37bΔ1-45) had significant inhibitory effects on the migration and invasion of endometrial carcinoma cells in vitro and effectively inhibited the metastasis of endometrial cancer cells in tumor-bearing nude mice. However, IL-37 did not affect the growth of endometrial carcinoma cells. Similarly, the expression of signal molecules in cell proliferation-related MAPK [25, 50] and PI3K/AKT [41] signal pathways had no obvious changes. Regretfully, only one cell line (Ishikawa cell) was used for the overexpression functional study, and only one cell line (AN3CA cell) was used for the knockdown study. Therefore, there are a few limitations. More endometrial carcinoma cells need to be studied in the future.
Tumor cell migration and invasion are related to EMT, cell motility, and degradation of basement membranes. To investigate the mechanism of IL-37bΔ1-45 affecting the migration and invasion of endometrial cancer cells, we detected the expression of EMT-related markers [34, 51], cell motility-related F-actin [35], and basement membrane degradation-related MMPs [25]. In our study, IL-37 did not affect EMT and F-actin in endometrial cancer cells. The discrepancy between our study and others may be because the function of IL-37 possesses tissue and cell specificity. Degradation of basement membranes is crucial for tumor cell invasion and metastasis [21, 52]. MMP-2 and -9, also known as the gelatinases, have been long recognized as major contributors to the ECM’s degradation during tumor invasion [39]. Here, we detected the effect of IL-37b on the expression of MMP2 and MMP9 in endometrial cancer cells. The results showed that the expression of MMP2 was downregulated in endometrial cancer cells after transfecting IL-37bΔ1-45 plasmid, and IL-37 knockdown could upregulate the levels of MMP2.
MMP2 is implicated in tumor metastasis and primary tumor growth; therefore, targeting MMP2 appears to offer highly specific means to inhibit basement membranes’ degradation. In our study, the MMP2-related NF-κB signal pathway [53] and Rac1 [54] were detected. The expression of the MMPs could be regulated at the transcriptional level via the NF-κB signal pathway. Rac1, a member of small GTPases, is well-characterized in the Rho family [55]. The GTP-bound form of Rac1 performs a mutual effect on downstream molecules and operates multiple cellular processes [56]. Previous reports suggested that Rac1 participated in the NF-κB signal pathway to promote tumor cell migration and invasion [57]. Li et al. [42] reported intracellular mature IL-37 directly bound to the CAAX motif in the C-terminal hypervariable region of Rac1 and then inhibited Rac1 membrane translocation and subsequent downstream signal pathway. Here, our result showed that IL-37bΔ1-45 could combine with Rac1-61L and inhibit the activation of Rac1, then downregulate the expression of p-P65 and MMP2. Rac1-61L overexpression in the IL-37bΔ1-45 stable expressed cells could reverse the inhibitory effect of IL-37, upregulating the levels of p-P65 and MMP2 and promoting the migration and invasion of endometrial cancer cells.
In conclusion, our research showed that IL-37 protein was significantly reduced in endometrioid adenocarcinoma cells, enhanced IL-37bΔ1-45 could inhibit the migration and invasion of endometrial cancer cells in vitro and in vivo by targeting Rac1/NF-κB/MMP2 signal pathway, suggesting intracellular mature IL-37b is a novel therapeutic target in endometrial cancer.
References
Shang Y. Molecular mechanisms of oestrogen and SERMs in endometrial carcinogenesis. Nat Rev Cancer. 2006;6:360–8.
Horn LC, Meinel A, Handzel R, Einenkel J. Histopathology of endometrial hyperplasia and endometrial carcinoma: an update. Ann Diagn Pathol. 2007;11:297–311.
Gien LT, Kwon JS, D’Souza DP, Radwan JS, Hammond JA, Sugimoto AK, et al. Brain metastases from endometrial carcinoma: a retrospective study. Gynecol Oncol. 2004;93:524–8.
Xiong S, Klausen C, Cheng JC, Leung PCK. TGFβ1 induces endometrial cancer cell adhesion and migration by up-regulating integrin αvβ3 via SMAD-independent MEK-ERK1/2 signaling. Cell Signal. 2017;34:92–101.
Sun XM, Dongol S, Qiu CP, Xu Y, Sun CG, Zhang ZW, et al. miR-652 promotes tumor proliferation and metastasis by targeting RORA in endometrial cancer. Mol Cancer Res. 2018;16:1927–39.
Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. Lancet. 2005;366:491–505.
Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15:10–7.
Mutter GL, Lin MC, Fitzgerald JT, Kum JB, Baak JP, Lees JA, et al. Altered PTEN expression as a diagnostic marker for the earliest endometrial precancers. J Natl Cancer Inst. 2000;92:924–30.
Myatt SS, Wang J, Monteiro LJ, Christian M, Ho KK, Fusi L, et al. Definition of microRNAs that repress expression of the tumor suppressor gene FOXO1 in endometrial cancer. Cancer Res. 2010;70:367–77.
Kumar S, McDonnell PC, Lehr R, Tierney L, Tzimas MN, Griswold DE, et al. Identification and initial characterization of four novel members of the interleukin-1 family. J Biol Chem. 2000;275:10308–14.
Boraschi D, Lucchesi D, Hainzl S, Leitner M, Maier E, Mangelberger D, et al. IL-37: a new anti-inflammatory cytokine of the IL-1 family. Eur Cytokine Netw. 2011;22:127–47.
Nicklin MJ, Barton JL, Nguyen M, FitzGerald MG, Duff GW, Kornman K. A sequence-based map of the nine genes of the human interleukin-1 cluster. Genomics. 2002;79:718–25.
Dinarello CA, Nold-Petry C, Nold M, Fujita M, Li S, Kim S, et al. Suppression of innate inflammation and immunity by interleukin-37. Eur J Immunol. 2016;46:1067–81.
Cavalli G, Dinarello CA. Suppression of inflammation and acquired immunity by IL-37. Immunol Rev. 2018;281:179–90.
Jia H, Liu J, Han B. Reviews of interleukin-37: functions, receptors, and roles in diseases. Biomed Res Int. 2018;2018:3058640.
Abulkhir A, Samarani S, Amre D, Duval M, Haddad E, Sinnett D, et al. A protective role of IL-37 in cancer: a new hope for cancer patients. J Leukoc Biol. 2017;101:395–406.
Quirk S, Agrawal DK. Immunobiology of IL-37: mechanism of action and clinical perspectives. Expert Rev Clin Immunol. 2014;10:1703–9.
Nold MF, Nold-Petry CA, Zepp JA, Palmer BE, Bufler P, Dinarello CA. IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol. 2010;11:1014–22.
Ding VA, Zhu Z, Xiao H, Wakefield MR, Bai Q, Fang Y. The role of IL-37 in cancer. Med Oncol. 2016;33:68.
Jiang M, Wang Y, Zhang H, Ji Y, Zhao P, Sun R, et al. IL-37 inhibits invasion and metastasis in non-small cell lung cancer by suppressing the IL-6/STAT3 signaling pathway. Thorac Cancer. 2018;9:621–9.
Zhang G, Tang C, Tan J, Liu R, Zhou M, Wu Z. IL-37 inhibits the proliferation, invasion and migration of SMMC-7721 cells in vitro. Chin J Cell Mol Immunol. 2015;31:1301–5.
Jiang JF, Deng Y, Xue W, Zheng TP, Sun AJ. Increased expression of interleukin 37 in the eutopic and ectopic endometrium of patients with ovarian endometriosis. Reprod Sci. 2016;23:244–8.
Kaabachi W, Kacem O, Belhaj R, Hamzaoui A, Hamzaoui K. Interleukin-37 in endometriosis. Immunol Lett. 2017;185:52–5.
Jiang J, Jiang Z, Xue M. Serum and peritoneal fluid levels of interleukin-6 and interleukin-37 as biomarkers for endometriosis. Gynecol Endocrinol. 2019;35:571–5.
Jiang J, Yu K, Jiang Z, Xue M. IL-37 affects the occurrence and development of endometriosis by regulating the biological behavior of endometrial stromal cells through multiple signaling pathways. Biol Chem. 2018;399:1325–37.
Fan YY, Chen HY, Chen W, Liu YN, Fu Y, Wang LN. Expression of inflammatory cytokines in serum and peritoneal fluid from patients with different stages of endometriosis. Gynecol Endocrinol. 2018;34:507–12.
Jiang JF, Xiao SS, Xue M. Decreased expression of interleukin-37 in the ectopic and eutopic endometria of patients with adenomyosis. Gynecol Endocrinol. 2018;34:83–6.
Wang S, An W, Yao Y, Chen R, Zheng X, Yang W, et al. Interleukin 37 expression inhibits STAT3 to suppress the proliferation and invasion of human cervical cancer cells. J Cancer. 2015;6:962–9.
Zaino RJ. FIGO staging of endometrial adenocarcinoma: a critical review and proposal. Int J Gynecol Pathol. 2009;28:1–9.
Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Am J Obstet Gynecol. 1975;122:262–3.
Ye Z, Wang C, Tang J, Zhou Y, Bai L, Liu Y, et al. Decreased interleukin-37 expression in Vogt-Koyanagi-Harada disease and upregulation following immunosuppressive treatment. J Interferon Cytokine Res. 2015;35:265–72.
Song L, Qiu F, Fan Y, Ding F, Liu H, Shu Q, et al. Glucocorticoid regulates interleukin-37 in systemic lupus erythematosus. J Clin Immunol. 2013;33:111–7.
Chen Z, Che Q, He X, Wang F, Wang H, Zhu M, et al. Stem cell protein Piwil1 endowed endometrial cancer cells with stem-like properties via inducing epithelial-mesenchymal transition. BMC Cancer. 2015;15:811.
Wu TJ, Xu B, Zhao GH, Luo J, Luo C. IL-37 suppresses migration and invasion of gallbladder cancer cells through inhibition of HIF-1alpha induced epithelial-mesenchymal transition. Eur Rev Med Pharmacol Sci. 2018;22:8179–85.
Acconcia F, Barnes CJ, Kumar R. Estrogen and tamoxifen induce cytoskeletal remodeling and migration in endometrial cancer cells. Endocrinology. 2006;147:1203–12.
Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62.
Ridley AJ. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006;16:522–9.
Najafi M, Farhood B, Mortezaee K. Extracellular matrix (ECM) stiffness and degradation as cancer drivers. J Cell Biochem. 2019;120:2782–90.
Bjorklund M, Koivunen E. Gelatinase-mediated migration and invasion of cancer cells. Biochim Biophys Acta. 2005;1755:37–69.
Huang N, Liu K, Liu J, Gao X, Zeng Z, Zhang Y, et al. Interleukin-37 alleviates airway inflammation and remodeling in asthma via inhibiting the activation of NF-κB and STAT3 signalings. Int Immunopharmacol. 2018;55:198–204.
Li TT, Zhu D, Mou T, Guo Z, Pu JL, Chen QS, et al. IL-37 induces autophagy in hepatocellular carcinoma cells by inhibiting the PI3K/AKT/mTOR pathway. Mol Immunol. 2017;87:132–40.
Li Y, Zhao M, Guo C, Chu H, Li W, Chen X, et al. Intracellular mature IL-37 suppresses tumor metastasis via inhibiting Rac1 activation. Oncogene. 2018;37:1095–106.
Ding VA, Zhu Z, Mantz AA, Xiao H, Wakefield MR, Bai Q, et al. The role of IL-37 in non-cancerous diseases. Pathol Oncol Res. 2017;23:463–70.
Osborne DG, Domenico J, Luo Y, Reid AL, Amato C, Zhai Z, et al. Interleukin-37 is highly expressed in regulatory T cells of melanoma patients and enhanced by melanoma cell secretome. Mol Carcinog. 2019;58:1670–9.
Yan X, Zhao J, Zhang R. Interleukin-37 mediates the antitumor activity in colon cancer through β-catenin suppression. Oncotarget. 2017;8:49064–75.
Kavlashvili T, Jia Y, Dai D, Meng X, Thiel KW, Leslie KK, et al. Inverse relationship between progesterone receptor and Myc in endometrial cancer. PloS One. 2016;11:e0148912.
Bommer I, Muzzio DO, Zygmunt M, Jensen F. Progesterone and estradiol exert an inhibitory effect on the production of anti-inflammatory cytokine IL-10 by activated MZ B cells. J Reprod Immunol. 2016;116:113–6.
Banchereau J, Pascual V, O’Garra A. From IL-2 to IL-37: the expanding spectrum of anti-inflammatory cytokines. Nat Immunol. 2012;13:925–31.
Ballak DB, van Diepen JA, Moschen AR, Jansen HJ, Hijmans A, Groenhof GJ, et al. IL-37 protects against obesity-induced inflammation and insulin resistance. Nat Commun. 2014;5:4711.
Carinci F, Lessiani G, Spinas E, Kritas SK, Ronconi G, Caraffa A, et al. Mast cell and cancer with special emphasis on il-37 an anti-inflammatory and inhibitor of innate immunity: new frontiers. J Biol Regul Homeost Agents. 2016;30:945–50.
Chen YH, Zhou BY, Wu XJ, Xu JF, Zhang JA, Chen YH, et al. CCL22 and IL-37 inhibit the proliferation and epithelial-mesenchymal transition process of NSCLC A549 cells. Oncol Rep. 2016;36:2017–24.
Chen YH, Zhou BY, Wu GC, Liao DQ, Li J, Liang SS, et al. Effects of exogenous IL-37 on the biological characteristics of human lung adenocarcinoma A549 cells and the chemotaxis of regulatory T cells. Cancer Biomarker. 2018;21:661–73.
Li X, Bao C, Ma Z, Xu B, Ying X, Liu X, et al. Perfluorooctanoic acid stimulates ovarian cancer cell migration, invasion via ERK/NF-κB/MMP-2/-9 pathway. Toxicol Lett. 2018;294:44–50.
Zhuge Y, Xu J. Rac1 mediates type I collagen-dependent MMP-2 activation. role in cell invasion across collagen barrier. J Biol Chem. 2001;276:16248–56.
Hakoshima T, Shimizu T, Maesaki R. Structural basis of the Rho GTPase signaling. J Biochem. 2003;134:327–31.
Zheng B, Ye L, Zhou Y, Zhu S, Wang Q, Shi H, et al. Epidermal growth factor attenuates blood-spinal cord barrier disruption via PI3K/Akt/Rac1 pathway after acute spinal cord injury. J Cell Mol Med. 2016;20:1062–75.
Gastonguay A, Berg T, Hauser AD, Schuld N, Lorimer E, Williams CL. The role of Rac1 in the regulation of NF-κB activity, cell proliferation, and cell migration in non-small cell lung carcinoma. Cancer Biol Ther. 2012;13:647–56.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Wang, X., Wei, Z., Tang, Z. et al. IL-37bΔ1-45 suppresses the migration and invasion of endometrial cancer cells by targeting the Rac1/NF-κB/MMP2 signal pathway. Lab Invest 101, 760–774 (2021). https://doi.org/10.1038/s41374-021-00544-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41374-021-00544-2