Abstract
Simultaneous administration of certain antihypertensive (renin–angiotensin system inhibitors and diuretics) and nonsteroidal anti-inflammatory drugs (NSAIDs) is associated with a renal toxicity syndrome known as “triple whammy” acute kidney injury (TW-AKI), yet poorly characterized at the pathophysiological level, as no specific experimental model exists on which to conduct preclinical research. Herein, we generated and characterized a rat model of TW-AKI (0.7 mg/kg/day trandolapril +400 mg/kg/day ibuprofen +20 mg/kg/day furosemide). Double treatments involving the NSAID caused a subclinical acute kidney injury, as they reduced glomerular filtration rate to a significant but not sufficient extent to increase Crpl concentration. Only the triple treatment generated an overt AKI with increased Crpl provided that animals were under partial water ingestion restriction. Histological examination revealed no evidence of tissue renal injury, and no proteinuria or makers of renal damage were detected in the urine. These findings, along with a normal fractional excretion of sodium and glucose, indicated that these drug combinations produce a prerenal type of AKI. In fact, blood pressure and renal blood flow were also reduced (most markedly following the triple combination), although renal dysfunction was more pronounced than expected for the corresponding pressure drop, supporting a key pathological role of the interference with renal autoregulation mechanisms. In summary, prerenal TW-AKI only occurs when volemia is challenged (i.e., by furosemide in partially water-deprived animals) under the effects of renin–angiotensin system inhibitors and NSAIDs. This model will facilitate further pathophysiological knowledge for a better diagnosis and clinical handling of this syndrome.
Similar content being viewed by others
Introduction
Hypertension is a highly prevalent and complex condition affecting 30% of the adult population worldwide [1], and a leading risk factor for cardiovascular morbidity and mortality [2]. In most patients, blood pressure (BP) control is not attained with monotherapy, and double and triple therapies are implemented despite concerns for increased side effects [3]. Diuretics and renin–angiotensin system (RAS) inhibitors (i.e., angiotensin-converting enzyme inhibitors, angiotensin type II receptor antagonists, and renin inhibitors) are among the five most prescribed families of antihypertensive drugs, along with calcium channel blockers and beta-blockers. Treated hypertensive patients often need analgesics for occasional or chronic pain, most commonly nonsteroidal anti-inflammatory drugs (NSAIDs), which exert their effect by inhibiting cyclooxygenase (COX), and thus the signaling and mediators produced downstream of COX, most relevantly vasoactive prostanoids [4, 5]. Indeed, 70% of the population in developed countries consumes analgesics regularly, mainly for headaches, joint pain, and fever [6]. Accordingly, NSAIDs are very often administered along with diuretics and RAS inhibitors, a combination increasingly associated with renal side effects. In particular, with a form of acute kidney injury (AKI) known as “triple whammy” (TW) AKI [7], which started to be recognized by the late 1990s and early 2000s [8,9,10].
AKI, a sudden loss of the kidneys excretory function, poses a serious health and economic concern with high incidence, growing by 10% yearly [11,12,13]. AKI accounts for 1–2% of hospital admissions and occurs in 2–7% of hospitalized patients, with dismal overall mortality rates of 23.9% in adults, 13.8% in children [14], and 50–80% among the critically ill [15,16,17,18]. The incidence of TW-AKI has been found to be up to 22% of patients receiving the three drugs simultaneously [19, 20]. Because of the high frequency of co-prescription, even low incidence rates would make TW a problem of great health and economic significance. TW-AKI incidence may even be higher than reported in controlled studies, because AKI is yet a poorly addressed of an issue within the community [21]. In fact, community-acquired AKI incidence might double or triple that of hospital-acquired AKI [22,23,24]. TW-AKI incidence is particularly likely to be underestimated, because of poor monitoring of renal function in hypertensive out-patients once prescribed NSAIDs or, worse, because of almost impossible monitoring after over-the-counter use and overuse [25].
TW-AKI is a clinical syndrome still poorly characterized at the pathophysiological level. TW-AKI is believed to result in part from the interference of RAS inhibitors and NSAIDs on the contractile role of renal afferent and efferent arterioles. Further exacerbating the condition is the autoregulatory demand imposed by diuretic-induced dehydration, giving rise to a hemodynamic, prerenal form of AKI [7]. Somewhat surprisingly, little (if any) experimental data are available to support or disprove this hypothesis, because thus far no animal model of TW-AKI exists. In this study, we generate, characterize and investigate the hemodynamics of a rat model of TW-AKI, which will serve as a basis for deepening into the study of this syndrome.
Materials and methods
Unless otherwise indicated, reagents were purchased from Sigma-Aldrich (Madrid, Spain).
Animals and experimental protocol
Male Wistar rats (200–250 g) were used and maintained under controlled conditions within the University of Salamanca Animal House facilities, with free access to water and standard chow. All procedures were approved by the Bioethics Committee of the University of Salamanca. Animals were handled according to the guidelines of the European Community Council Directive 2010/63/UE, and to the current Spanish legislation for experimental animal use and care, RD 53/2013. At specific time points, rats were placed in individual metabolic cages for urine collection. Rats were divided into 11 groups, which are described in Table 1. In all cases, oral dosage was adjusted daily according to water consumption. In a subset of experiments, animals were treated also like in group viii (I + T + F), but water intake was restricted to 15 mL per rat daily, by individual allocation, in order to induce partial dehydration.
Twenty-four-hour urine was collected in metabolic cages, cleared by centrifugation, and stored at −80 °C. Blood samples (200 μL) were obtained in heparinized capillaries from a small incision in the tail tip. Plasma was separated by centrifugation and kept at −80 °C. At the end, rats were anesthetized, blood was collected, and the kidneys were dissected. One half kidney was fixed in 3.7% para-formaldehyde for histological studies. The rest was frozen at −80 °C for further biochemical analysis.
Renal function and renal tissue assessment
Plasma creatinine (Crpl) and urea concentrations and urinary creatinine (Cru) were monitored with commercial kits (BioAssay, Hayward, USA). Creatinine clearance (ClCr) was calculated using the equation ClCr = (Cru × UF)/Crpl (were UF stands for urinary flow). Sodium concentration was measured in urine and blood samples using a specific sodium potentiometer (Horiba Scientific, Kyoto, Japan) and fractional excretion of sodium (FENa) were calculated using the equation FENa = [(Nau × Crpl)/(Napl × Cru)] × 100. Glucose concentration was measured in plasma samples using a specific glucose meter (Bayer, Barmen, Germany) and in urine samples with the O-toluidine method. Fractional excretion of glucose (FEGlc) was calculated using the equation FEGlc = [(Glcu × Crpl)/Glcpl × Cru) × 100]. Urine was also assayed for protein concentration with the Bradford method. Urinary biomarkers of renal damage were measured by western blot (as described below), except N-acetyl-beta-d-glucosaminidase activity, which was quantified with a commercial kit (Diazyme, Poway, CA, USA). For histopathological studies, paraffin blocks were made with 4% p-formaldehyde-fixed kidneys, and 2-μm tissue sections were stained with hematoxylin and eosin for visual inspection under the microscope. Tissue renal damage was scored in the main renal structures (glomeruli, renal tubules, renal interstitium, and blood vessels) in ten visual fields per sample.
Western blot
A volume of urine (μL) from each rat corresponding to the same excretion fraction (i.e., same % of their daily urinary output) was separated by acrylamide electrophoresis. Proteins were transferred to an Immobilon-P Transfer Membrane (Millipore, Madrid, Spain) and incubated with primary antibodies against the following proteins: neutrophil gelatinase-associated lipocalin (MBL International, Woburn, MA, USA), kidney injury molecule 1 (R&D systems, Minneapolis, MN, USA), fatty acid binding protein (Signalway Antibody, College Park, MD, USA), β2-microglobulin (Abcam, Cambridge, UK), fetuin A (Santa Cruz Biotechnology, Dallas, TX, USA), cystatin C (Santa Cruz Biotechnology, Dallas, TX, USA), nephrin (Novus Biologicals, Centennial, CO, USA), plasminogen activator inhibitor-1 (BD Transduction Laboratories, California, USA), followed by horseradish peroxidase-conjugated secondary antibodies and chemiluminescent detection (Immobilon Western Chemiluminescent HRP Substrate kit; Millipore, Madrid, Spain) with photographic films (Kodak, Madrid, Spain).
Blood pressure and renal blood flow measurement
Systolic BP was monitored in conscious rats by the tail-cuff method using a computerized sphygmomanometer (Cibertec, Madrid, Spain). Total renal blood flow (RBF) was measured before sacrifice in anesthetized animals with an ultrasonic probe (Transonic Systems, Ithaca, NY, USA) placed around the main renal artery. Cortical RBF was measured with a laser Doppler equipment (Moor Instruments, Axminster, UK).
Statistical analysis
All values are presented as mean ± SEM. Kolmogorov–Smirnov test was used to assess the normal distribution of data. For normally distributed data, student’ t tests or ANOVA tests were used, and Dunnett post hoc test was employed for multiple comparisons. For data nonconforming to a normal distribution, the Kruskal–Wallis test was used. p < 0.05 was considered statistically significant.
Results
Triple whammy induces an overt AKI and NSAID-including double whammies cause a subclinical AKI
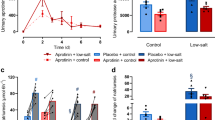
TW (trandolapril + ibuprofen + furosemide) is the only group that exhibits elevation in Crpl (Fig. 1a). In contrast, double whammies (DWs) (trandolapril + ibuprofen; trandolapril + furosemide; and ibuprofen + furosemide) and single treatments showing Crpl undistinguishable from controls. A group of rats treated with gentamicin were used as a positive control of intrinsic AKI (i.e., ATN), showing increased Crpl, urea and proteinuria, and decreased glomerular filtration rate (GFR), as previously reported [26, 27]. However, GFR estimated as ClCr was reduced in all groups treated with NSAID, and normal in all other groups (Fig. 1b and Supplementary Material Fig. 1). But marked differences can be discerned among NSAID-containing groups. The ibuprofen group (I) showed a statistically nonsignificant tendency towards GFR reduction. Ibuprofen-containing DWs showed a moderate reduction in GFR, while TW caused almost total and statistically significant loss of filtration. When only the RAS system is inhibited in the presence of the diuretic, GFR is mostly preserved; whereas when COX is inhibited (i.e., group I + F), GFR is cut to approximately half. In the absence of the diuretic challenge, the RAS inhibitor does not modify the effect of the NSAID (i.e., GFR in group I + T). No treatment group experienced proteinuria (Fig. 1c and Supplementary Material Fig. 1), except the gentamicin group, which had extensive proteinuria. Double treatments showed a nonstatistically significant reduction in urinary protein excretion, whereas TW rats underwent a marked, and statistically significant decrease in this parameter, with uncertain pathophysiological meaning.
Evolution of plasma creatinine concentration (a), creatinine clearance (as an estimation of glomerular filtration rate) at day 6 (b), and urinary protein excretion at day 6 (c). The evolution of c and d is detailed in Supplementary Fig. 1. Data represent the mean ± SEM of n = 6 (C), 3 (I), 3 (T), 3 (F), 14 (I + T), 14 (I + F), 14 (T + F), 29 (I + T + F), 7 (G). In a, the two-way ANOVA test was used. Within the same group: *p < 0.05 versus basal, and day 0. @p < 0.05 vs day 2. $p < 0.05 vs day 4. Within the same day: &p < 0.05 vs control, I, T and F. #p < 0.05 versus, I + T, I + F, and T + F. In b and c, the Kruskal–Wallis test was used. &p < 0.05 vs control. *p < 0.05 vs F. #p < 0.05 vs T + F. $p < 0.05 vs G. F furosemide, G gentamicin, I ibuprofen, T trandolapril.
Triple whammy induces a prerenal form of AKI
To determine the type of AKI induced by TW, we evaluated hematoxylin–eosin-stained renal specimens. Kidneys of rats treated with gentamicin were used as a model of intrinsic AKI amply reported in the literature [28, 29]. Figure 2 shows representative images of the renal cortex of control animals, and those treated with single, double and triple therapies, as well as with gentamicin. Representative images of the medulla and papilla are shown in Supplementary Material (Supplementary Material Fig. 2). All groups show intact glomeruli, tubuli, blood vessels and interstitium. Glomeruli show no evidence of mesangial proliferation or hyalinization. No signs of vacuolization, hypercellularity, or swelling are seen in the tubules, which keep unaltered epithelium and brush border. No hyaline material is observed in the lumen. The interstitium shows no stromal proliferation, inflammatory foci or fibrosis. No wall thickening, protein thrombi, or inflammation is evident in blood vessels. The only histological findings involve conformational changes in papillary tubuli in the T + F group, which become evident in the triple therapy group. Still, papillary tubules in this group show no evidence suggestive of injury.
We also measured in the urine a panel of known biomarkers of renal injury. Figure 3a summarizes the presence or absence of renal injury markers in the urine from the different experimental groups. This graph was constructed from average data for each biomarker in each group and time point, which are fully depicted in supplementary material Fig. 3. In short, the gentamicin group shows maximal urinary excretion for all kidney injury biomarkers, as expected from an ATN model, and as previously reported [26, 27], whereas only very mild, inconsistent, and scarce presence of some biomarkers is found in the urine of rats treated with trandolapril, furosemide, ibuprofen, and their combinations. This low excretion likely reflects the normal range of stochastic variation of these biomarkers, rather than true renal injury.
a Gray scale fingerprint of urinary biomarkers showing average levels derived from numerical values obtained by western blot analysis of the urine (detailed in Supplementary Fig. 3). Biomarker level in the gentamicin group were used as normalizing values (100%), to which biomarker values obtained in the other experimental groups were referred. b Fractional excretion of sodium (FENa) and c fractional excretion of glucose (FEGlc). Data represent the mean ± SEM of n = 6 (C), 3 (I), 3 (T), 3 (F), 14 (I + T), 14 (I + F), 14 (T + F), 29 (I + T + F), 7 (G). The two-way ANOVA test was used: *p < 0.05 versus all other times in the same group. #p < 0.05 versus all other groups on the same day. A.U. arbitrary units, F furosemide, G gentamicin, I ibuprofen, T trandolapril.
Absence of parenchymal injury was further supported by a normal FENa in all groups (Fig. 3b), except the ATN group (i.e., gentamicin), an index of tubular function traditionally used (with some limitations) to discriminate prerenal and renal AKI [30, 31]. FEGlc [32, 33], a similar index of tubular integrity, is also normal across the groups, but highly elevated in gentamicin-induced ATN (Fig. 3c). Altogether, these results indicate that TW-AKI lacks any morphological or biochemical signs of intrinsic AKI.
Triple whammy reduces blood pressure and renal blood flow
TW caused a marked drop in BP. Other treatments have a similar BP effect, albeit to a lesser extent (Fig. 4a). Figure 4b shows the relation of systolic BP and Crpl by treatment day 2. The values of Crpl in the range of 80 mmHg (i.e., about 40 mmHg drop from normal values) vary greatly from the TW to the H + M (hydralazine + minoxidil) groups, indicating that drugs interfering with the COX and RAS pathways (TW) alter GFR more than those that do not (H and M), for the same BP drop. In addition, and consistent with a handicapped renal autoregulation, total RBF was significantly and substantially reduced by TW, and to a lesser extent by DWs, whereas cortical RBF was less affected, although with the same pattern as total RBF (Fig. 5).
a Evolution of systolic blood pressure (SBP). b Scatter plot representation of paired plasma creatinine and SBP values. Data represent the mean ± SEM of n = 6 (C), 10 (I + T), 9 (I + F), 10 (T + F), 23 (I + T + F), 3 (M), 3 (M + H). In a, the multiple t-test, and in b, the two-way ANOVA test were used: Within the same group: *p < 0.05 versus basal. Çp < 0.05 vs day 0. &p < 0.05 vs day 2. $p < 0.05 vs day 4. Within the same day: #p < 0.05 versus the control. @p < 0.05 vs M. F furosemide, H hydralazine, I ibuprofen, M minoxidil, T trandolapril.
a Total renal blood flow measured by renal artery ultrasonography and b renal cortical blood flow measured by laser Doppler. c Representative images of renal cortical blood flow. Data represent the mean ± SEM of n = 6 (C), 10 (I + T), 8 (I + F), 9 (T + F), 7 (I + T + F). The Kruskal–Wallis test was used: *p < 0.05 versus the control. #p < 0.05 vs I + T. a.u. arbitrary units, F furosemide, I ibuprofen, T trandolapril.
Partial restriction in water ingestion is necessary for triple whammy AKI to occur
Ibuprofen-drinking rats show progressively lower daily water volume ingestion than rats that received no ibuprofen. This is due to the progressively increasing water odor caused by ibuprofen in solution. This observation prompted us to verify whether decreased water intake (i.e., some dehydration status) might be involved in the TW effect. In order to test this hypothesis, we changed daily the ibuprofen solution to reduce unpleasant odor. Consequently, rats then drank normal or near normal water volume. A new experiment was designed to control water intake under TW. Rats receiving the drugs (with ibuprofen solutions changed daily) were either allowed to drink ad libitum, or given 15 mL water daily per rat. As shown in Fig. 6, TW rats under partial water deprivation conditions showed an overt AKI, whereas rats drinking ad libitum showed no increase in Crpl. Average daily water consumption per rat was 25.0 ± 3.8 mL for rats drinking at libitum, and 10.8 ± 0.3 mL for rats under water deprivation (p < 0.05). These results suggest that TW is indeed a quadruple whammy in which the convergence of water deprivation (likely as a volemic stressor) plus the three drugs is necessary to induce an AKI.
Evolution of plasma creatinine concentration (Crpl) in TW animals allowed to drink ad libitum or under partial water intake restriction. Data represent the mean ± SEM of n = 3–4 per group. The multiple t-test was used: *p < 0.05 versus basal and day 4 values in the same group. #p < 0.05 versus TW allowed to drink ad libitum.
Discussion
Our study provides an experimental model of TW-AKI in the rat, which generates systemic and renal hemodynamic alterations in BP regulation and renal autoregulation mechanisms leading to sufficient GFR reduction to cause an increase in Crpl, with no gross injury to renal structures. In this model, the diuretic plays the role of any conditions, insults, stimuli or comorbidities that reduce volemia, thereby activating the hemodynamic regulation network to maintain BP and GFR. Thus, TW provides a model to study the effect of a hemodynamic challenge in animals in which the RAS and prostanoid systems are blocked. Importantly, our results show that TW occurs only in hemodynamically stressed rats (i.e., those with restricted water intake), which coincides with human studies showing that the highest incidence of TW-AKI occurs among the elderly [20], a significant fraction of whom are affected by dehydration [34].
According to internationally accepted scales (i.e., KDIGO, AKIN, and RIFLE) [35], no AKI occurs until Crpl increases. Renal injury or functional alterations not leading elevated Crpl are cataloged under “subclinical AKI,” such as that induced by DWs involving the NSAID, and even by ibuprofen alone. Due to increased tubular secretion, Crpl does not inversely parallel the decrease in GFR. It is not until creatinine secretion becomes saturated that further reduction in GFR translates into an increase in Crpl. Accordingly, GFR must be substantially reduced before Crpl increases and thus AKI occurs [36,37,38,39,40], which impacts clinical decision making. Double treatments (i.e., “double whammies”) involving an NSAID reduce GFR to a significant but not sufficient extent to increase Crpl concentration. And only TW generates an overt AKI, with prerenal signature. Understanding the differential underlying mechanisms of the subclinical and clinical stages in the continuum of each AKI form is of critical importance for timely, appropriate and personalized intervention.
As stated elsewhere [41], “subclinical AKI is still AKI.” In this regard, an interesting finding of this study is the recognition that, at least in rats, ibuprofen, and drug combinations including this NSAID, create a homologous, clinically relevant but under-recognized scenario which is hitherto difficult to detect at the population screening level, as no signs (e.g., Crpl) are evident. As a consequence of their diminished GFR and renal functional reserve, patients under ibuprofen might have an undetermined and hidden degree of risk of further renal damage and AKI, depending on their specific comorbidities, concomitant treatments, life style, and hydration status. These results also highlight the importance of the COX–prostaglandin axis for the maintenance of GFR under basal, but especially under hemodynamically challenging and demanding conditions such as those posed by simultaneous administration of other drugs, and potentially by other circumstances altering the homeostasis of the hemodynamic regulation network [7]. Under basal conditions, the RAS system has no significant participation in our model, congruently with its well-known role as a response mechanism. Following a hemodynamic challenge by a diuretic treatment, both the COX system and the RAS systems seem to be involved in the maintenance of BP and GFR, as it is necessary to inhibit both to cause an overt AKI. RAS inhibition has little effect on its own in basal conditions and under volemic reduction, because in the latter the role of RAS at maintaining GFR is superseded by further COX-mediated tone. This is revealed by the more pronounced effect of the NSAID in the presence than in the absence of trandolapril, upon forced diuresis.
Our results also shed light into systemic and renal hemodynamics regulation mechanisms. RBF and GFR are typically stabilized for mean BP ranging from about 80 to 180 mmHg, depending on the species (continuous black line in Fig. 7) [7]. This is achieved mainly through two renal autoregulatory mechanisms that accommodate afferent and efferent arteriole contraction and relaxation to maintain RBF and GFR constant upon variations of renal perfusion pressure (i.e., BP). These mechanisms are driven by (1) myogenic responses of the afferent arteriole; and (2) by the tubuloglomerular feedback (TGF), mediated in the macula densa by COX- and RAS-mediated processes (i.e., the targets for NSAIDs and RAS inhibitors, respectively). Without these mechanisms, GFR rises with BP (dotted line in Fig. 7), a proportional relationship that is predicted by computational models [42] to cross with the physiological GFR–BP curve approximately at the normal BP values region (Fig. 7). Because the myogenic mechanism is predicted to participate more in the response to BP increments [42], the TW pathophysiological scenario more importantly involves the TGF. In these circumstances, inhibition of both the COX and the RAS pathways (i.e., the macula densa-mediated autoregulation) substantially amplifies the impact of any BP drop on GFR (see gray line in Fig. 7). Because the therapeutic dosages of NSAID and RAS inhibitors likely do not completely block the response of the afferent and efferent arterioles, a certain degree of compensation remains, sufficient to counteract the hemodynamic challenge induced by furosemide. Thus, additional demand is necessary to overcome the remaining response capacity, and that demand is posed by partial dehydration. In some sense, the requirement of partial dehydration transforms the concept of TW into a quadruple whammy.
Inhibition of renal autoregulation has little impact on GFR when BP is within its normal range (Fig. 7; and I + T group in Fig. 1). It is thus necessary that some degree of BP reduction take place for TW-AKI (including subclinical TW-AKI) to occur. This BP drop will call upon RAS- and COX-mediated responses which will be interfered by the drugs. Table 1 summarizes the most important hemodynamic changes observed following dual and triple therapies. Of note, T + F and I + T groups induce a similar drop in BP, but T + F shows normal, whereas I + T reduced GFR, a difference that illustrates the impact of macula densa-mediated autoregulation on GFR. These results also suggest that TW drugs uncouple RBF from GFR regulation.
This study describes a rat model of TW-AKI, with prerenal, purely hemodynamic characteristics, and provides a better understanding of the pathophysiological mechanisms involved in the cooperative action of these drugs. Our results also highlight the key role of dehydration in the comorbidity for TW-AKI. This is also a model to further study and characterize the syndrome generated by single, dual, and triple drug treatments in different scenarios of comorbidity. They include hypertension, diabetes, dehydration, CKD, aging, and other circumstances challenging hemodynamics or altering some of the response mechanisms, and thus replacing the action of one or more drugs to precipitate AKI. For instance, single and double treatments of RAS inhibitors, diuretics and NSAIDs are also associated to some incidence of AKI, which could be explained by comorbid factors replacing the third drug, as this usually happens in senior patients with comorbidities. This leads to the concept of “multiple whammy” [7], which also awaits to be modeled and more deeply understood.
References
Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation. 2016;134:441–50.
Patel P, Ordunez P, DiPette D, Escobar MC, Hassell T, Wyss F, et al. Improved blood pressure control to reduce cardiovascular disease morbidity and mortality: the standardized hypertension treatment and prevention project. J Clin Hypertens. 2016;18:1284–94.
MacDonald TM, Williams B, Webb DJ, Morant S, Caulfield M, Cruickshank JK, et al. Combination therapy is superior to sequential monotherapy for the initial treatment of hypertension: a double-blind randomized controlled trial. J Am Heart Assoc. 2017;6:e006986.
Harirforoosh S, Jamali F. Renal adverse effects of nonsteroidal anti-inflammatory drugs. Expert Opin Drug Saf. 2009;8:669–81.
Bacchi S, Palumbo P, Sponta A, Coppolino MF. Clinical pharmacology of non-steroidal anti-inflammatory drugs: a review. Antiinflamm Antiallergy Agents Med Chem. 2012;11:52–64.
Abbott FV, Fraser MI. Use and abuse of over-the-counter analgesic agents. J Psychiatry Neurosci. 1998;23:13–34.
Prieto-García L, Pericacho M, Sancho-Martínez SM, Sánchez Á, Martínez-Salgado C, López-Novoa JM, et al. Mechanisms of triple whammy acute kidney injury. Pharmacol Ther. 2016;167:132–45.
Heerdink ER, Leufkens HG, Herings RMC, Ottervanger JP, Stricker BHC, Bakker A. NSAIDs associated with increased risk of congestive heart failure in elderly patients taking diuretics. Arch Intern Med. 1998;158:1108–12.
Boyd IW, Mathew TH, Thomas MC. COX-2 inhibitors and renal failure: the triple whammy revisited. Med J Aust. 2000;173:274.
Thomas MC. Diuretics, ACE inhibitors and NSAIDs—the triple whammy. Med J Aust. 2000;172:184–5.
Murugan R, Kellum JA. Acute kidney injury: what’s the prognosis? Nat Rev Nephrol. 2011;7:209–17.
Siew ED, Himmelfarb J. The inexorable rise of AKI: can we bend the growth curve? J Am Soc Nephrol. 2013;24:3–5.
Chan L, Mehta S, Chauhan K, Poojary P, Patel S, Pawar S, et al. National trends and impact of acute kidney injury requiring hemodialysis in hospitalizations with atrial fibrillation. J Am Heart Assoc. 2016;5:e004509. pii
Susantitaphong P, Cruz DN, Cerda J, Abulfaraj M, Alqahtani F, Koulouridis I, et al. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol. 2013;8:1482–93.
Neild GH. Multi-organ renal failure in the elderly. Int Urol Nephrol. 2001;32:559–65.
Block CA, Schoolwerth AC. The epidemiology and outcome of acute renal failure and the impact on chronic kidney disease. Semin Dial. 2006;19:450–4.
Kellum JA, Hoste EAJ. Acute kidney injury: epidemiology and assessment. Scand J Clin Lab Investig. 2008;68:6–11.
Kerr M, Bedford M, Matthews B, O’donoghue D. The economic impact of acute kidney injury in England. Nephrol Dial Transplant. 2014;29:1362–8.
Lapi F, Azoulay L, Yin H, Nessim SJ, Suissa S. Concurrent use of diuretics, angiotensin converting enzyme inhibitors, and angiotensin receptor blockers with non-steroidal anti-inflammatory drugs and risk of acute kidney injury: nested case-control study. BMJ. 2013;346:e8525.
Fournier J-P, Sommet A, Durrieu G, Poutrain JC, Lapeyre-Mestre M, Montastruc JL, et al. More on the “Triple Whammy”: antihypertensive drugs, non-steroidal anti-inflammatory agents and acute kidney injury—a case/non-case study in the French pharmacovigilance database. Ren Fail. 2014;36:1166–8.
Blakeman T, Harding S, O’Donoghue D. Acute kidney injury in the community: why primary care has an important role. Br J Gen Pract. 2013;63:173–4.
Schissler MM, Zaidi S, Kumar H, Deo D, Brier ME, McLeish KR. Characteristics and outcomes in community-acquired versus hospital-acquired acute kidney injury. Nephrology. 2013;18:183–7.
Der Mesropian PJ, Kalamaras JS, Eisele G, Phelps KR, Asif A, Mathew RO. Long-term outcomes of community-acquired versus hospital-acquired acute kidney injury: a retrospective analysis. Clin Nephrol. 2014;81:174–84.
Wonnacott A, Meran S, Amphlett B, Talabani B, Phillips A. Epidemiology and outcomes in community-acquired versus hospital-acquired aki. Clin J Am Soc Nephrol. 2014;9:1007–14.
Cryer B, Barnett MA, Wagner J, Wilcox CM. Overuse and misperceptions of nonsteroidal anti-inflammatory drugs in the United States. Am J Med Sci. 2016;352:472–80.
Quiros Y, Ferreira L, Sancho-Martínez SM, González-Buitrago JM, López-Novoa JM, López-Hernández FJ. Sub-nephrotoxic doses of gentamicin predispose animals to developing acute kidney injury and to excrete ganglioside M2 activator protein. Kidney Int. 2010;78:1006–15.
Ferreira L, Quiros Y, Sancho-Martínez SM, García-Sánchez O, Raposo C, López-Novoa JM, et al. Urinary levels of regenerating islet-derived protein III β and gelsolin differentiate gentamicin from cisplatin-induced acute kidney injury in rats. Kidney Int. 2011;79:518–28.
Lopez-Novoa JM, Quiros Y, Vicente L, Morales AI, Lopez-Hernandez FJ. New insights into the mechanism of aminoglycoside nephrotoxicity: an integrative point of view. Kidney Int. 2011;79:33–45.
Quiros Y, Vicente-Vicente L, Morales AI, López-Novoa JM, López-Hernández FJ. An integrative overview on the mechanisms underlying the renal tubular cytotoxicity of gentamicin. Toxicol Sci. 2011;119:245–56.
Perazella MA, Coca SG. Traditional urinary biomarkers in the assessment of hospital-acquired AKI. Clin J Am Soc Nephrol. 2012;7:167–74.
Vanmassenhove J, Glorieux G, Hoste E, Dhondt A, Vanholder R, Van Biesen W. Urinary output and fractional excretion of sodium and urea as indicators of transient versus intrinsic acute kidney injury during early sepsis. Crit Care. 2013;17:R234.
Trumper L, Monasterolo LA, Ochoa E, Elias MM. Tubular effects of acetaminophen in the isolated perfused rat kidney. Arch Toxicol. 1995;69:248–52.
Arndt C, Morgenstern B, Hawkins D, Wilson D, Liedtke R, Miser J. Renal function following combination chemotherapy with ifosfamide and cisplatin in patients with osteogenic sarcoma. Med Pediatr Oncol. 1999;32:93–96.
Frangeskou M, Lopez-Valcarcel B, Serra-Majem L. Dehydration in the elderly: a review focused on economic burden. J Nutr Health Aging. 2015;19:619–27.
Thomas ME, Blaine C, Dawnay A, Devonald MA, Ftouh S, Laing C, et al. The definition of acute kidney injury and its use in practice. Kidney Int. 2015;87:62–73.
Pfaller W, Gstraunthaler G. Nephrotoxicity testing in vitro-what we know and what we need to know. Environ Health Perspect. 1998;106 Suppl 2:559–69.
Sharma A, Mucino MJ, Ronco C. Renal functional reserve and renal recovery after acute kidney injury. Nephron Clin Pract. 2014;127:94–100. S. Karger AG
Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem. 1992;38:1933–53.
Emeigh Hart SG. Assessment of renal injury in vivo. J Pharmacol Toxicol Methods. 2005;52:30–45.
Solomon R, Segal A. Defining acute kidney injury: what is the most appropriate metric? Nat Clin Pract Nephrol. 2008;4:208–15.
Ronco C, Kellum JA, Haase M. Subclinical AKI is still AKI. Crit Care. 2012;16:313.
Sgouralis I, Layton AT. Theoretical assessment of renal autoregulatory mechanisms. Am J Physiol Renal Physiol. 2014;306:F1357–71.
Acknowledgements
This study was supported by grants from the Government of Spain [Instituto de Salud Carlos III (PI14/01776, DT15S/00166, PI15/01055 and PI18/00996, PI19/00588 and Retic RD016/0009/0025, REDINREN), Ministerio de Economía y Competitividad (IPT-2012-0779-010000)], FEDER funds, the Canada 150 Research Chair program and the NSERC Discovery grant program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Prieto-García, L., Vicente-Vicente, L., Blanco-Gozalo, V. et al. Pathophysiological mechanisms underlying a rat model of triple whammy acute kidney injury. Lab Invest 100, 1455–1464 (2020). https://doi.org/10.1038/s41374-020-0473-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41374-020-0473-9
This article is cited by
-
Impact of nephrotoxic drugs on urinary biomarkers of renal function in very preterm infants
Pediatric Research (2022)
-
Drug Interactions Affecting Kidney Function: Beware of Health Threats from Triple Whammy
Advances in Therapy (2022)