Abstract
The deregulation of splicing factors and alternative splicing are increasingly viewed as major contributory factors in tumorigenesis. In this study, we report overexpression of a key splicing factor, heterogeneous nuclear ribonucleoprotein A2B1 (HNRNPA2B1), and thereby misregulation of alternative splicing, which is associated with the poor prognosis of head and neck cancer (HNC). The role of HNRNPA2B1 in HNC tumorigenesis via deregulation of alternative splicing is not well understood. Here, we found that the CRISPR/Cas9-mediated knockout of HNRNPA2B1 results in inhibition of HNC cells growth via the misregulation of alternative splicing of MST1R, WWOX, and CFLAR. We investigated the mechanism of HNRNPA2B1-mediated HNC cells growth and found that HNRNPA2B1 plays an important role in the alternative splicing of a proto-oncogene, macrophage stimulating 1 receptor (MST1R), which encodes for the recepteur d’origine nantais (RON), a receptor tyrosine kinase. Our results indicate that HNRNPA2B1 mediates the exclusion of cassette exon 11 from MST1R, resulting in the generation of RON∆165 isoform, which was found to be associated with the activation of Akt/PKB signaling in HNC cells. Using the MST1R-minigene model, we validated the role of HNRNPA2B1 in the generation of RON∆165 isoform. The depletion of HNRNPA2B1 results in the inclusion of exon 11, thereby reduction of RON∆165 isoform. The decrease of RON∆165 isoform causes inhibition of Akt/PKB signaling, which results in the upregulation of E-cadherin and downregulation of vimentin leading to the reduced epithelial-to-mesenchymal transition. The overexpression of HNRNPA2B1 in HNRNPA2B1 knockout cells rescues the expression of the RON∆165 isoform and leads to activation of Akt/PKB signaling and induces epithelial-to-mesenchymal transition in HNC cells. In summary, our study identifies HNRNPA2B1 as a putative oncogene in HNC that promotes Akt/PKB signaling via upregulation of RON∆165 isoform and promotes epithelial to mesenchymal transition in head and neck cancer cells.
Similar content being viewed by others
Introduction
Head and neck cancer (HNC) is a heterogeneous disease, comprising of tumors that arise from the lip, oral cavity, hypopharynx, oropharynx, nasopharynx, or larynx. HNC is one of the leading causes of cancer-associated deaths and is expected to rise by 30% in 2020 [1]. HNC is considered one of the highly aggressive malignancies, at eighth place worldwide [2, 3]. The annual incidence of HNC worldwide is predicted to be more than 550,000 cases, with around 300,000 associated deaths [4, 5]. In Southeast Asian countries, particularly in India, the incidences of HNC occurrence are reported to be high among the male population [1, 4], associated with late diagnosis and poor prognosis [6]. Despite having improved treatment modalities, the advanced stage HNC control rate is just 40% for 5 years [7], and the overall survival rate of HNC patients remains poor [8]. This indicates the urgent need for the identification of new molecular therapeutic targets.
The heterogeneous nuclear ribonucleoproteins (HNRNP) represent a large family of RNA-binding proteins that are expressed in almost all tissue types [9]. These proteins majorly regulate the process of alternative splicing [10] and are also reported to be involved in mRNA trafficking, transcriptional regulation, and translation [9, 11, 12]. The investigation of HNRNPs role in the regulation of gene expression is receiving increased interest. Interestingly, various members of HNRNP family are reported with deregulated expression in many diseases, including cancer. Moreover, few of the HNRNPs are investigated for their role in growth and development of cancer cells [13, 14]. The heterogeneous nuclear ribonucleoprotein A2B1 (HNRNPA2B1) is one of the highly expressing cellular proteins [9] and reported to be involved in mRNA splicing, trafficking, and mRNA translation [15,16,17]. Intriguingly, the overexpression of HNRNPA2B1 has been observed in various malignancies [18,19,20] and shown to promote oncogenesis via regulating the transcription as well as alternative splicing of multiple cancer-specific isoforms [17, 21, 22]. Recent reports have shown that the process of alternative splicing is widely dysregulated in cancer cells and promotes the expression of cancer-specific isoforms that remains absent in normal cells [21, 23,24,25,26,27,28,29]. The changes in alternative splicing due to aberrant expression of splicing regulators are evolving as the basis of tumorigenesis, metastasis, and chemotherapeutic resistance thus holds a strong potential to be exploited for therapeutic development.
The HNRNPA2B1 has been shown to affect the process of alternative splicing of various genes and promotes the expression of cancer-specific isoforms [30]. Similarly, the macrophage stimulating 1 receptor (MST1R), which encodes for recepteur d’origine nantais (RON), is reported to have multiple alternatively spliced isoforms [31,32,33]. The MST1R is a member of the MET family of receptor tyrosine kinases, which is observed to be deregulated in various malignancies [34,35,36] and positively correlates with tumor aggressiveness and metastasis [37]. However, MST1R validation as a molecular target has remained a challenge due to production of various isoform in cancer cells, which remains absent in normal cells. Notably, one of the cancer-specific isoforms of MST1R is generated by exclusion of exon 11 in the final transcript, which is known as RON∆165. The expression of RON∆165 isoform is reported in different cancers, including breast, ovarian, pancreatic, and colon cancer [33, 38, 39]. The RON∆165 is known for self-activation and constitutively signals for activation of various downstream signaling pathways, which in turn promotes the invasive behavior of cancer cells and ultimately promotes cellular transformation [40, 41]. Previous reports have identified the role of mutation and a handful of splicing regulators that impacts the splicing of exon 11 in the final transcript [42,43,44,45]. These studies clearly indicate that the splicing of MST1R and expression of RON∆165 is highly regulated, and the role of the splicing regulator is partially understood. Several preclinical studies have highlighted MST1R as a potential target for anticancer therapy [46, 47]. While complete understanding of the MST1R splicing mechanism and the regulatory proteins might help us to identify novel drug targets.
In this study, we have investigated the role of HNRNPA2B1 in head and neck oncogenesis. We observed that HNRNPA2B1 exhibits a high degree of expression in HNC tumor tissue compared with paired normal. Interestingly, we observed that increased expression of HNRNPA2B1 modulates the alternative splicing of MST1R and promotes the expression of RON∆165 isoform. Further observation identified that RON∆165 isoform of MST1R well correlated with the increased activation of the Akt/PKB pathway and epithelial-to-mesenchymal transition (EMT) by increasing the expression of a mesenchymal marker, vimentin, and decreasing the expression of epithelial marker E-cadherin. Conclusively, our study shows that HNRNPA2B1–MST1R–Akt/PKB axis is crucial for cancer cell growth and can be exploited for the development of new molecular therapeutics.
Materials and methods
HNC sample collection
The HNC tissue and paired normal tissue were obtained from Bansal Hospital. After surgery, tumor and adjacent normal tissue pairs were collected and immediately snap-frozen and stored at −80 °C until further use. Informed consent was obtained from patients undergoing surgery for HNC at Bansal Hospital, Bhopal, India. This study was approved by the Institute Ethics Committee. Clinical characteristics of patients used in the study are presented in Supplementary Tables S1 and S2.
Cell culture
Human HNC cell lines H413 (ECACC 06092007) and H157 (ECACC 07030901) were obtained from the European Collection of Authenticated Cell Culture (ECACC, Salisbury UK). The cell line was cultured in the ECACC recommended media, supplemented with 10% fetal bovine serum (Thermo Fisher Scientific, USA), 100 units/ml of penicillin and streptomycin (Thermo Fisher Scientific, Waltham, USA), 2 mM l-glutamine (Sigma, Saint Louis, USA), and 0.5 µg/ml sodium hydrocortisone succinate (Sigma, Saint Louis, USA). The cell line was cultured in a humidified atmosphere at 37 °C and 5% CO2.
Cell viability assay/MTT assay
HNRNPA2B1-depleted H413 cells were seeded in 96-well culture plates (4 × 103 cell/well) and cultured for 0, 12, 24, and 48 h (in triplicate for each condition). Post incubation, the 10 µl MTT (Sigma, Saint Louis, USA) stock solution (2 mg/ml) was added to each well and incubated for 2 h in the dark. Post incubation, formazan crystals formed in the cells were solubilized using dimethyl sulfoxide, and the optical density was analyzed at 570 and 750 nm using plate reader BioTek Eon (BioTek, Winooski, USA)
Wound-healing assay
The HNRNPA2B1-depleted H413 cells were seeded in a 12-well plate (1 × 105 cells/well), and the cells were cultured till 100% confluency. The wound was created using 200 μl pipette tip, sequentially, the cells were washed with 1× PBS for two times to remove cellular debris. The wounds were visualized at 10× with an inverted microscope (Olympus, Tokyo, Japan), and three random images were captured for indicated time points. The wound width was measured using Q-Capture software and the graph was plotted with GraphPad Prism5 (La Jolla, CA, USA).
Transwell invasion assay
The transwell invasion assay was performed as described earlier [48]. Briefly, the HNRNPA2B1-depleted, control, and HNRNPA2 overexpressing H413 cells were serum-starved for 24 h and followed for invasion assay. The H413 (2 × 104) cells were resuspended in serum-free media and added to the upper chamber of transwell (Corning, NY, USA) over Matrigel (Corning, Bedford, MA, USA) layer and the lower chamber was filled with 10% FBS containing media. The cells were incubated for 24 h in cell culture incubator. The nonmigrated cells in the upper layer of Matrigel were removed, and cells migrated to the lower chamber of transwell were fixed in 4% paraformaldehyde, stained with 0.05% crystal violet, and five random fields were counted using an inverted microscope (Olympus, Tokyo, Japan).
Quantitative real-time reverse transcriptase-PCR (qRT-PCR)
Total RNA was extracted from cultured H413 and H157, HNC cells using Trizol (Thermo Fisher Scientific, Waltham, USA) according to the manufacturer’s instruction. RNA was quantified using BioSpectrometer (Eppendorf, Hamburg, Germany). The 1 µg of total RNA was reverse transcribed by Superscript complementary DNA (cDNA) synthesis kit (Thermo Fisher Scientific, Waltham, USA) as per the manufacturer’s instructions. The experiment was done using SYBR green (Promega, Fitchburg, Wisconsin, USA) with light cycler 480 II (Roche, Basel, Switzerland) according to the manufacturer’s protocol. Primers were designed using the IDT Primer Quest tool (https://www.idtdna.com/). Primers used in this study are mentioned in Supplementary Table S3. The average cycle thresholds of independent experiments were calculated and normalized to housekeeping control gene RPS16 for HNC cells using the formula [2(Ct control − Ct target)]. The Student’s t test was followed to compare gene expression between two different groups. P < 0.05 was considered as statistically significant.
Immunoblotting
The HNC tissues were first crushed and powdered by following liquid nitrogen-mediated freezing in a mortar pestle. The powdered cells were lysed in NP-40 lysis buffer. Similarly, the H413 and H157, HNC cells were trypsinized and washed with ice-cold 1X PBS and lysed with NP-40 lysis buffer. The protein was quantified using Bradford assay and was separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride (PVDF) membrane (Millipore, Burlington, USA). The protein carrying PVDF membranes were blocked with 5% skimmed milk and probed with different primary antibodies: anti-HNRNPA2B1 (Abcam, Melbourne, Australia), anti-GAPDH (Cell Signaling Technology, Beverly, MA, USA), anti-phosphoAkt (Cell Signaling Technology, Beverly, USA), anti-pan Akt (Cell Signaling Technology, Beverly, USA), anti-E-cadherin (Abcam, Melbourne, Australia), anti-vimentin (Abcam, Melbourne, Australia), anti-ZEB1 (Cell Signaling Technology, Beverly, MA, USA), anti-SLUG (Cell Signaling Technology, Beverly, MA, USA), anti-TWIST2 (Sigma, Saint Louis, USA), and anti-Flag (Novus Biologicals, CO, USA) in a 1:1000 dilution. After 2 h of primary antibody incubation at room temperature, membranes were washed with 1× TBST (tris-buffered saline and Tween-20) three times and incubated with secondary antibodies Alexa-Flour 680 anti-rabbit IgG (Thermo Fisher Scientific, Waltham, MA, USA) and Alexa-Flour 790 goat anti-mouse IgG (Thermo Fisher Scientific, Waltham, MA, USA) for 45 min at room temperature, in dark. The membrane was washed with 1X TBST three times, and bands were visualized using the Odyssey membrane scanning system (Li-Cor Biosciences, Bad Homburg, Germany).
Immunohistochemistry (IHC)
The tissue specimens were sectioned using a manual microtome and fixed on a poly l-lysine coated slide. Post antigen retrieval, the sections were stained with an anti-HNRNPA2B1 antibody (1:100; Abcam, Melbourne, Australia) for 2 h in a wet chamber. Post incubation, the Vectastain kit (PK-8200, Burlingame, CA, USA) protocol was followed as per the manufacturer’s protocol. The degree of immunostaining was reviewed by an unbiased pathologist and scored semiquantitatively. The staining index divided as: + as week, ++ as moderate, and +++ as strong staining.
Cloning
The HNRNPA2 overexpression plasmid was constructed by amplifying the hnRNPA2 fragment from H413 cells cDNA using Q5 Hotstart High-Fidelity DNA Polymerase (New England Biolabs, MA, USA). The primers used for amplification are mentioned in Supplementary Table S3. The hnRNPA2 product was cloned between the EcoRI and XhoI sites in the PCMV-3Tag1A expression vector (Agilent Technologies, Santa Clara, CA, USA).
Construction of dual chromatic MST1R minigene
The MST1R/RON gene region from exon 10 to 12, including the introns, was selected for the minigene construction. MST1R/RON gene was PCR amplified from human genomic DNA (HEK293) in two halves. The first half extended from exon 10 to middle of exon 11 (75th bp). EcoRI restriction site and start codon were placed before exon 10. An extra base pair was inserted after the 75th bp of exon 11 in the first half such that if exon 11 is included, it will result in reading frameshift. The second half extended from 76th bp of exon 11 to exon 12. The two halves of RON were joined by overlap extension PCR (OE-PCR). The eGFP gene was PCR amplified from pEGFP-n1 vector without the initiation codon. A stop codon and BamHI restriction site were inserted at the end of eGFP gene through PCR. RON and eGFP genes were sewed together by OE-PCR. The entire RON-GFP construct was cloned into EcoRI/BamHI site of pmCherry-N1 vector (Supplementary Fig. S4a). The primers used for MST1R-minigene construction are mentioned in Supplementary Table S3.
HNRNPA2B1 knockout
The HNRNPA2B1 specific guide RNA was identified using Genetic Perturbation Platform, Broad Institute, and cloned in pLentiCRISPR-E vector (78852, Addgene, Watertown, USA) by following the Zhang Lab short guide RNA cloning protocol. The HNRNPA2B1guide RNA cloned pLentiCRISPR-E vector was transfected in H413 and H157, HNC cells. Post transfection, cells were cultured and selected with 1 µg/ml puromycin for 5 days. The selected cells were cultured and used for downstream applications.
Oncomine and MiPanda data analysis
The expression of HNRNPA2B1 was analyzed using the Oncomine database (https://www.oncomine.org/resource/login.html) as well as in MiPanda database (http://www.mipanda.org), and among various cancers, HNC profiles were selected for further investigation. The expression of HNRNPA2B1 was analyzed in HNC normal and tumor tissues as per the experimental requirement. The analyzed expression data and graphs were exported for representation.
Survival analysis
The data of survival correlation with HNRNPA2B1 expression was obtained from the protein atlas database (https://www.proteinatlas.org/). The expression of HNRNPA2B1 was analyzed in HNC tissues as well as in other cancer types. The data was classified into low-expression and high-expression groups, and Kaplan–Meier survival curve analysis was followed.
Densitometric analysis
Densitometric analysis was performed using ImageJ software suit. Briefly, the band intensity was calculated with ImageJ and normalized with a respective loading control. The normalized control values were further normalized to one, and the protein fold change was calculated using control values.
Statistical analysis
The statistical analysis in this study was performed with GraphPad Prism5 (La Jolla, CA, USA). The differences between the two groups were compared using an unpaired two-tailed Student’s t test. The differences between three or more groups were calculated using a one-way analysis of variance by the Newman–Keuls test. The differences were considered statistically significant with *P < 0.05, **P < 0.01, and ***P < 0.001, ns nonsignificant difference (P > 0.05).
Results
HNRNPA2B1 is overexpressed in HNC and confers poor clinical outcome
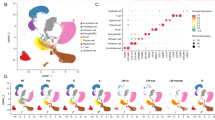
To understand the effect of HNRNPA2B1 in HNC, we analyzed the expression of HNRNPA2B1 in HNC profiles using The Cancer Genome Atlas (TCGA) and MiPanda database, which showed the significant overexpression of HNRNPA2B1 in tumor samples (Fig. 1a, b). To further strengthen our observation, we analyzed HNRNPA2B1 expression in few more HNC profiles using the Oncomine database, which showed consistent observation with significantly increased expression of HNRNPA2B1 in head and neck tumor tissue in comparison with normal tissue (Fig. 1c–e and Supplementary Fig. S1a).
HNRNPA2B1 expression analysis in different head and neck cancer profiles. a HNRNPA2B1 expression analysis using RNAseq database MiPanda. b HNRNPA2B1 expression analysis using the TCGA dataset. HNRNPA2B1 expression analysis using Oncomine database (c) Peng, (d) Cromer, and (e) Estilo. f Immunoblotting of HNRNPA2B1 in head and neck cancer tumor and paired normal samples. g Immunohistochemistry analysis for the expression as well as localization of HNRNPA2B1 in head and neck cancer tumor samples. Scale bar = 125 µm. TPM transcript per million, H & E hematoxylin and eosin.
Furthermore, the HNRNPA2B1 expression was validated at the protein level by immunoblotting and IHC in HNC tumor and paired normal tissues. The immunoblotting and IHC showed highly increased expression of HNRNPA2B1 (Fig. 1f, g and Supplementary Fig. S1b, c), which was consistent with RNA profile expression. Interestingly, we also noticed that HNRNPA2B1 expression and its cytoplasmic localization positively correlated with higher-grade HNC in comparison with low-grade HNC (Fig. 1g and Supplementary Fig. S1c). This observation indicates that HNRNPA2B1 cytoplasmic localization might be critical for cancer advancement. Moreover, we also observed that higher expression of HNRNPA2B1 correlates with poor survival of HNC patients (Supplementary Fig. S1d) and the negative correlation of HNRNPA2B1 expression with patient survival is not limited to HNC but includes liver, renal, and prostate cancer as well (Supplementary Fig. S1e). Collectively, we observed that HNRNPA2B1 is overexpressed and positively correlates with higher grades of HNC and confers poor prognosis.
HNRNPA2B1 promotes cellular proliferation, migration, and invasion of HNC cells
The elevated expression of HNRNPA2B1 in the HNC tumor sample prompted us to investigate its role in tumorigenesis. To understand the role of HNRNPA2B1 in HNC tumorigenesis, we depleted the expression of HNRNPA2B1 in H413 HNC cells using CRISPR/Cas9 (Fig. 2a). Sequentially, we identified the involvement of HNRNPA2B1 in cellular proliferation via MTT assay. We observed a significant reduction in cellular proliferation in HNRNPA2B1-depleted cells in comparison with control cells (Fig. 2b). Our observation was consistent with the previous reports wherein HNRNPA2B1 overexpression is reported to promote breast and cervical cancer cell proliferation [49]. Notably, previous reports indicated a possible role of HNRNPA2B1 in invasion and migration of breast cancer and pancreatic cancer cells [49, 50]; however, the role of HNRNPA2B1 in migration and invasion of HNC cell remained unexplored. Hence, we investigated the invasive and migratory behavior of HNRNPA2B1-depleted HNC cells through Matrigel invasion assay and wound-healing assay, respectively. We observed a significantly compromised migration and invasive behavior of HNRNPA2B1-depleted HNC cells (Fig. 2c, d). These results indicate that HNRNPA2B1 promotes cellular proliferation, cell migration, and invasion in HNC cells, and thereby promotes head and neck tumorigenesis.
a Immunoblotting of HNRNPA2B1 in HNRNPA2B1-depleted head and neck cancer cell line, H413. b Relative cellular proliferation analyzed using MTT assay in HNRNPA2B1-depleted H413 cells. (n = 3). c Cell migration was analyzed through wound-healing assay, (left) wound was observed under the microscope, scale bar 500 µm, and (right) quantification of wound width (n = 3). d The cell invasion was analyzed via Matrigel invasion assay, (left) single field of invaded cells was captured under the microscope, scale bar: 250 µm, and (right) invaded cells were counted at five different fields under the microscope (n = 3).
HNRNPA2B1 promotes EMT via alternative splicing of MST1R
The HNRNPA2B1 is shown to promote the oncogenesis via regulating alternative splicing [51, 52]. Hence, to understand the role of HNRNPA2B1 in alternative splicing of few of the target genes [53] in HNC, we analyzed the differential splicing pattern of WWOX, CFLAR, and MST1R in HNC cells. We observed an enhanced expression of the full-length isoforms of target genes, WWOX (inclusion of exon 6–8) [53], CFLAR (exclusion and inclusion of exon 7) [54], and MST1R (inclusion of exon 11) upon HNRNPA2B1 depletion (Fig. 3a, b, d). Notably, we observed much-pronounced effect of HNRNPA2B1 on MST1R splicing in comparison with other candidate genes; hence, we focused more on investigating the effect of MST1R splicing switch on HNC tumorigenesis.
Semiquantitative PCR to analysis of HNRNPA2B1 target gene. a WWOX exon 6–8 inclusion/skipping. b CFLAR exon 7 inclusion/skipping. c Pictorial diagram to show differential splicing pattern of MST1R in HNC cells. d MST1R exon 11 inclusion/skipping. e MST1R-minigene transfection and fluorescence analysis in control and HNRNPA2B1-depleted H413 HNC cells, oral cancer cells. Scale bar = 200 µm. f Immunoblotting of HNRNPA2B1, pAkt, pan Akt, E-cadherin, vimentin, and GAPDH in control and HNRNPA2B1-depleted H413 cells.
In order to understand the effect of HNRNPA2B1 overexpression on MST1R in HNC cells, first, we analyzed the expression of MST1R in primary HNC tissues, metastatic cancer tissue, and HNC cell lines with the help of MiPanda database (Supplementary Fig. S2a). We observed a significant upregulation of MST1R in primary and metastatic HNC tissues as well as in HNC cell lines in comparison with normal tissue. Similarly, we also analyzed the expression of MST1R in HNRNPA2B1-depleted cells (Supplementary Fig. S2b) through qRT-PCR, which showed significant downregulation of MST1R. Secondly, in addition to decreased expression of MST1R, we also observed differential expression of multiple isoforms of MST1R [32] in HNRNPA2B1-depleted HNC cells. The MST1R is reported to have two major isoforms, normal-specific isoform with the inclusion of exon 11 and cancer-specific isoform with the exclusion of exon 11 also known as RON∆165 [31]. The upregulation of RON∆165 isoform of MST1R has been reported in various cancers [39]. Hence, we investigated the MST1R splicing switch upon HNRNPA2B1 depletion. We performed the MST1R semiquantitative PCR using the exon 9 and exon 12 primers and observed a dramatic reduction in RON∆165 isoform of MST1R upon HNRNPA2B1 depletion in H413 HNC cells (Fig. 3c, d). In addition, we also validated the MST1R splicing switch in another HNRNPA2B1-depleted HNC cells, H157 (Supplementary Fig. S2c) and observed a splicing pattern similar to H413 cells (Supplementary Fig. S2d). Moreover, we observed a similar splicing switch when we used the dual chromatic MST1R-minigene system. The MST1R-minigene transfection showed the consistent result, higher expression of exon 11 included isoform indicated by expression of mCherry protein and negligible expression of exon 11 excluded isoform or RON∆165, indicated by the expression of green fluorescent protein in HNRNPA2B1-depleted HNC cells, H413 and vice versa in wild-type HNC cells (Fig. 3e).
The expression of HNRNPA2B1 has been reported to have a positive correlation with invasive behavior [55] and EMT in cancer cells [50]; however, how the HNRNPA2B1 increases the invasive behavior of cancer cells and how it promotes EMT in HNC remained poorly understood. Interestingly, the RON∆165 isoform of MST1R is known to affect the various cellular signaling, including Akt/PKB signaling [56]. Hence, we hypothesized that HNRNPA2B1 might be regulating the EMT via promoting the expression of oncogenic isoforms, RON∆165 of MST1R. Therefore, we analyzed the activation of Akt/PKB signaling upon HNRNPA2B1 depletion and found that HNRNPA2B1-depleted cells showed dramatically reduced activation of Akt/PKB signaling, analyzed via pAkt immunoblotting (Fig. 3f). Next, we hypothesized that HNRNPA2B1 might be promoting the EMT via the activation of Akt/PKB pathway. Hence, we analyzed the expression of EMT regulators TWIST2, SLUG, and ZEB1 (Supplementary Fig. S2e), as well as, the epithelial marker E-cadherin and mesenchymal marker, vimentin (Fig. 3f) in H413 HNC cells. Similarly, we also analyzed the expression of vimentin in another hnRNA2B1 knockout HNC cells, H157 (Supplementary Fig. S2c). We observed a dramatic reduction in the expression of EMT regulators and markers in both the HNC cell lines; additionally, we also examined the expression of TWIST2 in the same HNC patients’ samples, used to analyze HNRNPA2B1 expression and observed an elevated expression in tumor samples in comparison with paired normal tissues (Supplementary Fig. S2f). Collectively, these results indicate that HNRNPA2B1 promotes EMT in HNC cells via MST1R splicing dependent activation of Akt/PKB pathway.
Ectopic expression of HNRNPA2B1 restores the oncogenic potential of HNC cells and promotes EMT
In order to validate the effects of HNRNPA2B1 depletion on HNC, we planned to ectopically express the majorly expressed isoform, HNRNPA2, in HNRNPA2B1-depleted H413 HNC cells. The expression of Flag-tagged HNRNPA2 was confirmed by immunoblotting (Fig. 4a). Post validation, we analyzed the differential splicing pattern of MST1R through semiquantitative PCR and found the expected restored expression of RON∆165 isoform (Fig. 4b). Similarly, we confirmed the MST1R splicing switch using the MST1R-minigene system and observed an increased expression of exon 11 excluded, RON∆165 of MST1R shown by expression of green fluorescent protein (Fig. 4c). We observed in Fig. 3 that HNRNPA2B1-depleted cells showed reduced expression of MST1R, so we tested the expression of MST1R in HNRNPA2 expressing cells, and interestingly, we observed that the ectopic expression of HNRNPA2 also restored the expression of MST1R (Supplementary Fig. 3a).
a Immunoblotting of Flag-tagged HNRNPA2 in HNRNPA2B1-depleted H413 cells. b Semiquantitative PCR analysis of alternative splicing of MST1R upon HNRNPA2B1 depletion as well as, upon ectopic expression of Flag-tagged HNRNPA2 in HNRNPA2B1-depleted H413 cells. c MST1R-minigene transfection and fluorescence analysis in control cells, HNRNPA2B1-depleted with vector control cells, and HNRNPA2B1-depleted HNRNPA2 expressing HNC cells. Scale bar = 200 µm. d Immunoblotting of HNRNPA2B1, pAkt, pan Akt, E-cadherin, vimentin, and GAPDH in control cells, HNRNPA2B1-depleted and HNRNPA2B1-depleted HNRNPA2 expressing H413 cells. e Relative cellular proliferation analyzed using MTT assay in HNRNPA2B1-depleted and HNRNPA2 reexpressing H413 cells (n = 3). f The cell invasion was analyzed via Matrigel invasion assay, (left) single field of invaded cells was captured under the microscope, scale bar: 250 µm, and (right) invaded cells were counted at five different fields under the microscope (n = 3).
Furthermore, to prove our hypothesis of HNRNPA2B1 regulated MST1R splicing dependent activation of the Akt/PKB pathway (Supplementary Fig. 3b), we analyzed the Akt/PKB signaling via immunoblotting of pAkt and observed the restored activation of Akt/PKB signaling (Fig. 4d) in HNRNPA2-expressing but HNRNPA2B1-depleted H413 cells. Similarly, the expression of vimentin was also found to be upregulated, while the expression of E-cadherin was significantly reduced upon HNRNPA2 expression in HNRNPA2B1-depleted cells (Fig. 4d). Sequentially, we validated the restored oncogenic potential via analyzing the cellular proliferation and invasive behavior of HNC cells. We observed the restored cellular proliferation of HNRNPA2B1-depleted HNC cells upon HNRNPA2 reexpression, which was comparable with control cells (Fig. 4e). Similarly, we also observed restored invasive behavior of HNRNPA2 expressing cells in comparison with control H413 HNC cells (Fig. 4f). Altogether, these results indicated that the HNRNPA2B1–MST1R–Akt/PKB axis is crucial for HNC cell growth and invasive behavior; wherein, HNRNPA2B1 regulates MST1R splicing and promotes the cellular proliferation and invasion via activation of Akt/PKB pathway.
Discussion
The deregulated alternative splicing events have been observed in various malignancies [57], which support all the major hallmarks of cancer, including cellular proliferation, apoptosis, chemotherapeutic resistance, and immune evasion [58]. The interlink between deregulated alternative splicing and tumor onset and progression is still emerging as a novel and vital facet of cancer biology. Our data showed in this study directly present a link of a contributory role for MST1R splicing as part of a common oncogenic splicing switch regulated by RNA-binding protein HNRNPA2B1 in HNC cells.
The deregulated expression of HNRNPA2B1 is observed in various malignancies [59, 60] and this observation is consistent in HNC cells as well. Moreover, the correlation of HNRNPA2B1 expression and its cytoplasmic localization with aggressive lung cancer is proposed as an effective prognosis factor for lung cancer [61], and interestingly, we also observed higher cytoplasmic localization of HNRNPA2B1 in high-grade HNC tissues. This clearly establishes that HNRNPA2B1 not only overexpresses in various cancer types but also follows a similar mode of oncogenesis. Moreover, the higher expression of HNRNPA2B1 is well correlated with various properties of cancer cells such as proliferation, migration invasion, and metabolic rewiring [50, 62,63,64,65]; however, the complete understanding of underlying molecular mechanisms remained poorly understood. While, in our study, we showed that HNRNPA2B1 affects proliferation and invasive behavior of HNC cells, which adds a new dimension to a plethora of HNRNPA2B1-mediated oncogenesis. Recent pieces of evidence have shown the role of HNRNPA2B1 in promoting the invasive behavior of breast and cervical cancer cells via hyperactivation of various signaling, including ERK-STAT3 and Akt/PI3K pathways [49, 63], which ultimately upregulates the expression of transcription factors of EMT pathway such as SNAIL and TWIST2. However, the effect of HNRNPA2B1 overexpression on the activation of various signaling remained unanswered. Our attempt to answer this question fills up the missing link wherein we showed that elevated expression of HNRNPA2B1 encourages the splicing switch of MST1R and partially, promotes the expression of MST1R as well. The increased expression of MST1R regulates various receptor tyrosine kinase signaling [66].
Recent advancement in splicing biology indicates that cancer cells reframe the expression of various genes, including splicing regulators [67], which might also affect the process of alternative splicing [68]. The HNRNPA2B1 is well investigated for its canonical role as a splicing regulator via promoting the expression of cancer-specific isoforms, which affects the various cellular pathways such as metabolism, apoptosis, proliferation, and invasion [52, 53, 69]. The differential alternative splicing of WWOX, CFLAR, and MST1R upon HNRNPA2B1 overexpression in HNC has broadened the current knowledge of HNRNPA2B1 splicing targets in HNC cells. The MST1R encodes for a receptor tyrosine kinase and shown to have an expression of multiple isoforms in various cancer cells [70, 71]. As reported previously, the alternative splicing of MST1R leads to the expression of RON∆165 isoform which in turn leads to the constitutive activation of downstream signaling [70, 72]. Our study showed that elevated expression of HNRNPA2B1 regulates MST1R splicing switch and encourages the expression of RON∆165. The RON∆165 isoform expression promotes constitutive activation of Akt/PKB pathway in HNC cells. However, complete understanding of HNRNPA2B1-mediated splicing switch of MST1R still remains a question. Recent reports have indicated that the expression as well as differentially spliced isoforms of MST1R affect cellular motility in different malignancies and regulates various downstream signaling, which ultimately affects the process of EMT in cancer cells [73, 74]. Accumulated evidence indicates that the cancer cells undergo EMT show more aggressive growth and metastasis [75]. In our report, we observed the reversal of EMT upon depletion of HNRNPA2B1, which was evident by the decreased expression of vimentin and increased expression of E-cadherin. As the MST1R inhibition reported ineffective due to its multiple spliced forms [32], HNRNPA2B1 stands a possibility to be used as an alternative target for the better management of aggressive tumor types. Moreover, HNRNPA2B1 depletion and complementation experiments indicate that the phenomenon of EMT is being governed by HNRNPA2B1 in HNC cells, which correlates well with the association of HNRNPA2B1 with high-grade tumor tissues of hepatic cancer [61] and HNC. This clearly indicates that HNRNPA2B1 is one of the key regulators of EMT in different malignancies.
Conclusively, in this study, we observed the elevated expression of HNRNPA2B1 in HNC tumor tissues. Similarly, our observation reiterated with the fact that higher-grade cancers have more cytoplasmic localization of HNRNPA2B1 than the lower-grade cancers. In an attempt to understand the underlying molecular signaling of HNRNPA2B1, we revealed that HNRNPA2B1 is involved in the splicing of a proto-oncogene MST1R and promotes the generation of RON∆165 isoform in HNC cells. We identified the Akt/PKB signaling as one of the targets of MST1R splicing switch in HNC cells, which is reported for its hyperactivation in cancer cells [76]. Consequentially, we also observed high expression of EMT markers (low E-cadherin and high vimentin) in HNRNPA2B1-expressing cells than in HNRNPA2B1-deficient cells. As an outcome of changes in cancer-signaling pathways on the cellular phenotypes, we compared cell viability, migration, and the invasive behavior of HNRNPA2B1-sufficient and HNRNPA2B1-deficient cells. We concluded that overexpression of HNRNPA2B1 makes the cells proliferate, migrate, and invade faster than normal, which are the hallmarks of oncogenesis (Fig. 5). These results suggest that inhibition of HNRNPA2B1 might compromise the invasive and aggressive behavior of HNC cells. Altogether, we reveal a crucial role for HNRNPA2B1 in MST1R splicing-switch-mediated EMT, invasion, and migration in HNC cells and speculate that HNRNPA2B1 may qualify as a potential therapeutic target.
The HNRNPA2B1 higher expression promotes the MST1R exon 11 skipping and leads to the expression of cancer-specific isoform which in turn promotes the hyperactivation of Akt/PKB pathway. Sequentially, the activation of Akt/PKB pathway promotes cellular EMT via regulating the E-cadherin and vimentin expression and increases invasive behavior of HNC cells.
References
Gupta B, Johnson NW. Oral cancer: Indian pandemic. Br Dent J. 2017;222:497.
Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11:9–22.
Massano J, Regateiro FS, Januario G, Ferreira A. Oral squamous cell carcinoma: review of prognostic and predictive factors. Oral Surg, Oral Med, Oral Pathol, Oral Radiol, Endodontics. 2006;102:67–76.
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
Chaturvedi AK, Anderson WF, Lortet-Tieulent J, Curado MP, Ferlay J, Franceschi S, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol. 2013;31:4550–9.
Gupta A, Ajith A, Singh S, Panday RK, Samaiya A, Shukla S. PAK2-c-Myc-PKM2 axis plays an essential role in head and neck oncogenesis via regulating Warburg effect. Cell Death Dis. 2018;9:825–15.
Cocks H, Ah-See K, Capel M, Taylor P. Palliative and supportive care in head and neck cancer: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol. 2016;130:S198–S207.
Narayanan SP, Singh S, Gupta A, Yadav S, Singh SR, Shukla S. Integrated genomic analyses identify KDM1A’s role in cell proliferation via modulating E2F signaling activity and associate with poor clinical outcome in oral cancer. Cancer Lett. 2015;367:162–72.
Dreyfuss G, Kim VN, Kataoka N. Messenger-RNA-binding proteins and the messages they carry. Nat Rev Mol Cell Biol. 2002;3:195–205.
Black DL. Mechanisms of alternative pre-messenger RNA splicing. Annu Rev Biochem. 2003;72:291–336.
Keene JD. RNA regulons: coordination of post-transcriptional events. Nat Rev Genet. 2007;8:533–43.
Glisovic T, Bachorik JL, Yong J, Dreyfuss G. RNA-binding proteins and post-transcriptional gene regulation. FEBS Lett. 2008;582:1977–86.
Qiao L, Xie N, Bai Y, Li Y, Shi Y, Wang J, et al. Identification of upregulated HNRNPs associated with poor prognosis in pancreatic cancer. Biomed Res Int. 2019;2019:5134050–11.
Alsagaby SA. Transcriptomics-based validation of the relatedness of heterogeneous nuclear ribonucleoproteins to chronic lymphocytic leukemia as potential biomarkers of the disease aggressiveness. Saudi Med J. 2019;40:328–38.
Park SJ, Lee H, Jo DS, Jo YK, Shin JH, Kim HB, et al. Heterogeneous nuclear ribonucleoprotein A1 post-transcriptionally regulates Drp1 expression in neuroblastoma cells. Biochim Biophys Acta. 2015;1849:1423–31.
Alarcon CR, Goodarzi H, Lee H, Liu X, Tavazoie S, Tavazoie SF. HNRNPA2B1 is a mediator of m(6)A-dependent nuclear RNA processing events. Cell. 2015;162:1299–308.
Cloutier A, Shkreta L, Toutant J, Durand M, Thibault P, Chabot B. hnRNP A1/A2 and Sam68 collaborate with SRSF10 to control the alternative splicing response to oxaliplatin-mediated DNA damage. Sci Rep. 2018;8:2206–14.
Zhou J, Nong L, Wloch M, Cantor A, Mulshine JL, Tockman MS. Expression of early lung cancer detection marker: hnRNP-A2/B1 and its relation to microsatellite alteration in non-small cell lung cancer. Lung Cancer. 2001;34:341–50.
Barcelo C, Etchin J, Mansour MR, Sanda T, Ginesta MM, Sanchez-Arevalo Lobo VJ, et al. Ribonucleoprotein HNRNPA2B1 interacts with and regulates oncogenic KRAS in pancreatic ductal adenocarcinoma cells. Gastroenterology. 2014;147:882–92 e8.
Qu XH, Liu JL, Zhong XW, Li XI, Zhang QG. Insights into the roles of hnRNP A2/B1 and AXL in non-small cell lung cancer. Oncol Lett. 2015;10:1677–85.
Guha M, Srinivasan S, Guja K, Mejia E, Garcia-Diaz M, Johnson FB, et al. HnRNPA2 is a novel histone acetyltransferase that mediates mitochondrial stress-induced nuclear gene expression. Cell Discov. 2016;2:16045–16.
Stockley J, Villasevil ME, Nixon C, Ahmad I, Leung HY, Rajan P. The RNA-binding protein hnRNPA2 regulates beta-catenin protein expression and is overexpressed in prostate cancer. RNA Biol. 2014;11:755–65.
Venables JP, Klinck R, Koh C, Gervais-Bird J, Bramard A, Inkel L, et al. Cancer-associated regulation of alternative splicing. Nat Struct Mol Biol. 2009;16:670–6.
Xi L, Feber A, Gupta V, Wu M, Bergemann AD, Landreneau RJ, et al. Whole genome exon arrays identify differential expression of alternatively spliced, cancer-related genes in lung cancer. Nucleic Acids Res. 2008;36:6535–47.
Roy M, Xu Q, Lee C. Evidence that public database records for many cancer-associated genes reflect a splice form found in tumors and lack normal splice forms. Nucleic Acids Res. 2005;33:5026–33.
Karni R, de Stanchina E, Lowe SW, Sinha R, Mu D, Krainer AR. The gene encoding the splicing factor SF2/ASF is a proto-oncogene. Nat Struct Mol Biol. 2007;14:185–93.
Cooper TA, Wan L, Dreyfuss G. RNA and disease. Cell. 2009;136:777–93.
Singh S, Narayanan SP, Biswas K, Gupta A, Ahuja N, Yadav S, et al. Intragenic DNA methylation and BORIS-mediated cancer-specific splicing contribute to the Warburg effect. Proc Natl Acad Sci USA. 2017;114:11440–5.
Yadav S, Bhagat SD, Gupta A, Samaiya A, Srivastava A, Shukla S. Dietary-phytochemical mediated reversion of cancer-specific splicing inhibits Warburg effect in head and neck cancer. BMC Cancer. 2019;19:1031–15.
Wang MH, Lee W, Luo YL, Weis MT, Yao HP. Altered expression of the RON receptor tyrosine kinase in various epithelial cancers and its contribution to tumourigenic phenotypes in thyroid cancer cells. J Pathol. 2007;213:402–11.
Krishnaswamy S, Mohammed AK, Tripathi G, Alokail MS, Al-Daghri NM. Splice variants of the extracellular region of RON receptor tyrosine kinase in lung cancer cell lines identified by PCR and sequencing. BMC Cancer. 2017;17:738–7.
Krishnaswamy S, Bukhari I, Mohammed AK, Amer OE, Tripathi G, Alokail MS, et al. Identification of the splice variants of Recepteur d’Origine nantais (RON) in lung cancer cell lines. Gene. 2018;679:335–40.
Mayer S, Hirschfeld M, Jaeger M, Pies S, Iborra S, Erbes T, et al. RON alternative splicing regulation in primary ovarian cancer. Oncol Rep. 2015;34:423–30.
Camp ER, Yang A, Gray MJ, Fan F, Hamilton SR, Evans DB, et al. Tyrosine kinase receptor RON in human pancreatic cancer: expression, function, and validation as a target. Cancer. 2007;109:1030–9.
Maggiora P, Marchio S, Stella MC, Giai M, Belfiore A, De Bortoli M, et al. Overexpression of the RON gene in human breast carcinoma. Oncogene. 1998;16:2927–33.
Zhou D, Pan G, Zheng C, Zheng J, Yian L, Teng X. Expression of the RON receptor tyrosine kinase and its association with gastric carcinoma versus normal gastric tissues. BMC Cancer. 2008;8:353–7.
Thomas RM, Toney K, Fenoglio-Preiser C, Revelo-Penafiel MP, Hingorani SR, Tuveson DA, et al. The RON receptor tyrosine kinase mediates oncogenic phenotypes in pancreatic cancer cells and is increasingly expressed during pancreatic cancer progression. Cancer Res. 2007;67:6075–82.
Ghigna C, Giordano S, Shen H, Benvenuto F, Castiglioni F, Comoglio PM, et al. Cell motility is controlled by SF2/ASF through alternative splicing of the Ron protooncogene. Mol Cell. 2005;20:881–90.
Chakedis J, French R, Babicky M, Jaquish D, Mose E, Cheng P, et al. Characterization of RON protein isoforms in pancreatic cancer: implications for biology and therapeutics. Oncotarget. 2016;7:45959–75.
Chakedis J, French R, Babicky M, Jaquish D, Howard H, Mose E, et al. A novel protein isoform of the RON tyrosine kinase receptor transforms human pancreatic duct epithelial cells. Oncogene. 2016;35:3249–59.
Collesi C, Santoro MM, Gaudino G, Comoglio PM. A splicing variant of the RON transcript induces constitutive tyrosine kinase activity and an invasive phenotype. Mol Cell Biol. 1996;16:5518–26.
Moon H, Cho S, Loh TJ, Zhou J, Ghigna C, Biamonti G, et al. A 2-nt RNA enhancer on exon 11 promotes exon 11 inclusion of the Ron proto-oncogene. Oncol Rep. 2014;31:450–5.
Braun S, Enculescu M, Setty ST, Cortes-Lopez M, de Almeida BP, Sutandy FXR, et al. Decoding a cancer-relevant splicing decision in the RON proto-oncogene using high-throughput mutagenesis. Nat Commun. 2018;9:3315–18.
Lefave CV, Squatrito M, Vorlova S, Rocco GL, Brennan CW, Holland EC, et al. Splicing factor hnRNPH drives an oncogenic splicing switch in gliomas. EMBO J. 2011;30:4084–97.
Bonomi S, di Matteo A, Buratti E, Cabianca DS, Baralle FE, Ghigna C, et al. HnRNP A1 controls a splicing regulatory circuit promoting mesenchymal-to-epithelial transition. Nucleic Acids Res. 2013;41:8665–79.
Logan-Collins J, Thomas RM, Yu P, Jaquish D, Mose E, French R, et al. Silencing of RON receptor signaling promotes apoptosis and gemcitabine sensitivity in pancreatic cancers. Cancer Res. 2010;70:1130–40.
Wang Q, Quan H, Zhao J, Xie C, Wang L, Lou L. RON confers lapatinib resistance in HER2-positive breast cancer cells. Cancer Lett. 2013;340:43–50.
Kumari P, Saha I, Narayanan A, Narayanan S, Takaoka A, Kumar NS, et al. Essential role of HCMV deubiquitinase in promoting oncogenesis by targeting anti-viral innate immune signaling pathways. Cell Death Dis. 2017;8:e3078.
Hu Y, Sun Z, Deng J, Hu B, Yan W, Wei H, et al. Splicing factor hnRNPA2B1 contributes to tumorigenic potential of breast cancer cells through STAT3 and ERK1/2 signaling pathway. Tumour Biol. 2017;39:1010428317694318.
Dai S, Zhang J, Huang S, Lou B, Fang B, Ye T, et al. HNRNPA2B1 regulates the epithelial-mesenchymal transition in pancreatic cancer cells through the ERK/snail signalling pathway. Cancer Cell Int. 2017;17:12.
Chen M, Zhang J, Manley JL. Turning on a fuel switch of cancer: hnRNP proteins regulate alternative splicing of pyruvate kinase mRNA. Cancer Res. 2010;70:8977–80.
David CJ, Chen M, Assanah M, Canoll P, Manley JL. HnRNP proteins controlled by c-Myc deregulate pyruvate kinase mRNA splicing in cancer. Nature. 2010;463:364–8.
Golan-Gerstl R, Cohen M, Shilo A, Suh SS, Bakacs A, Coppola L, et al. Splicing factor hnRNP A2/B1 regulates tumor suppressor gene splicing and is an oncogenic driver in glioblastoma. Cancer Res. 2011;71:4464–72.
Fricker N, Beaudouin J, Richter P, Eils R, Krammer PH, Lavrik IN. Model-based dissection of CD95 signaling dynamics reveals both a pro- and antiapoptotic role of c-FLIPL. J Cell Biol. 2010;190:377–89.
Wang H, Liang L, Dong Q, Huan L, He J, Li B, et al. Long noncoding RNA miR503HG, a prognostic indicator, inhibits tumor metastasis by regulating the HNRNPA2B1/NF-kappaB pathway in hepatocellular carcinoma. Theranostics. 2018;8:2814–29.
Benight NM, Waltz SE. Ron receptor tyrosine kinase signaling as a therapeutic target. Expert Opin Ther Targets. 2012;16:921–31.
Oltean S, Bates DO. Hallmarks of alternative splicing in cancer. Oncogene. 2014;33:5311–8.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Dowling P, Pollard D, Larkin A, Henry M, Meleady P, Gately K, et al. Abnormal levels of heterogeneous nuclear ribonucleoprotein A2B1 (hnRNPA2B1) in tumour tissue and blood samples from patients diagnosed with lung cancer. Mol Biosyst. 2015;11:743–52.
Zhou ZJ, Dai Z, Zhou SL, Hu ZQ, Chen Q, Zhao YM, et al. HNRNPAB induces epithelial-mesenchymal transition and promotes metastasis of hepatocellular carcinoma by transcriptionally activating SNAIL. Cancer Res. 2014;74:2750–62.
Cui H, Wu F, Sun Y, Fan G, Wang Q. Up-regulation and subcellular localization of hnRNP A2/B1 in the development of hepatocellular carcinoma. BMC Cancer. 2010;10:356.
Li L, Yang Y, Wu M, Yu Z, Wang C, Dou G, et al. Beta-Asarone induces apoptosis and cell cycle arrest of human glioma U251 cells via suppression of HnRNP A2/B1-mediated pathway in vitro and in vivo. Molecules. 2018;23:1072.
Shi X, Ran L, Liu Y, Zhong SH, Zhou PP, Liao MX, et al. Knockdown of hnRNP A2/B1 inhibits cell proliferation, invasion and cell cycle triggering apoptosis in cervical cancer via PI3K/AKT signaling pathway. Oncol Rep. 2018;39:939–50.
Brandi J, Cecconi D, Cordani M, Torrens-Mas M, Pacchiana R, Dalla Pozza E, et al. The antioxidant uncoupling protein 2 stimulates hnRNPA2/B1, GLUT1 and PKM2 expression and sensitizes pancreas cancer cells to glycolysis inhibition. Free Radic Biol Med. 2016;101:305–16.
Pan H, Luo C, Li R, Qiao A, Zhang L, Mines M, et al. Cyclophilin A is required for CXCR4-mediated nuclear export of heterogeneous nuclear ribonucleoprotein A2, activation and nuclear translocation of ERK1/2, and chemotactic cell migration. J Biol Chem. 2008;283:623–37.
Yao HP, Zhou YQ, Zhang R, Wang MH. MSP-RON signalling in cancer: pathogenesis and therapeutic potential. Nat Rev Cancer. 2013;13:466–81.
Nebbioso A, Tambaro FP, Dell’Aversana C, Altucci L. Cancer epigenetics: moving forward. PLoS Genet. 2018;14:e1007362:1–25.
Kornblihtt AR. Epigenetics at the base of alternative splicing changes that promote colorectal cancer. J Clin Investig. 2017;127:3281–3.
Clower CV, Chatterjee D, Wang Z, Cantley LC, Vander Heiden MG, Krainer AR. The alternative splicing repressors hnRNP A1/A2 and PTB influence pyruvate kinase isoform expression and cell metabolism. Proc Natl Acad Sci USA. 2010;107:1894–9.
Zhou YQ, He C, Chen YQ, Wang D, Wang MH. Altered expression of the RON receptor tyrosine kinase in primary human colorectal adenocarcinomas: generation of different splicing RON variants and their oncogenic potential. Oncogene. 2003;22:186–97.
Eckerich C, Schulte A, Martens T, Zapf S, Westphal M, Lamszus K. RON receptor tyrosine kinase in human gliomas: expression, function, and identification of a novel soluble splice variant. J Neurochem. 2009;109:969–80.
Yao HP, Zhuang CM, Zhou YQ, Zeng JY, Zhang RW, Wang MH. Oncogenic variant RON160 expression in breast cancer and its potential as a therapeutic target by small molecule tyrosine kinase inhibitor. Curr Cancer Drug Targets. 2013;13:686–97.
Wang J, Li L, Liu S, Zhao Y, Wang L, Du G. FOXC1 promotes melanoma by activating MST1R/PI3K/AKT. Oncotarget. 2016;7:84375–87.
Kim SA, Lee KH, Lee DH, Lee JK, Lim SC, Joo YE, et al. Receptor tyrosine kinase, RON, promotes tumor progression by regulating EMT and the MAPK signaling pathway in human oral squamous cell carcinoma. Int J Oncol. 2019;55:513–26.
Mittal V. Epithelial mesenchymal transition in tumor metastasis. Annu Rev Pathol. 2018;13:395–412.
Manning BD, Toker A. AKT/PKB signaling: navigating the network. Cell. 2017;169:381–405.
Acknowledgements
This work was supported by the Wellcome Trust/Department of Biotechnology (DBT) India Alliance Fellowship grant IA/I/16/2/502719 and Board of Research in Nuclear Sciences (BRNS) (37(1)/14/30/2016-BRNS). AG was supported by a fellowship from IISER Bhopal, SY was supported by a fellowship from Centre for Scientific and Industrial Research (CSIR), and APT was supported by a fellowship from the Department of Science & Technology (DST). We also thank all members of the Epigenetics and RNA Processing Lab for their helpful discussions and technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Gupta, A., Yadav, S., PT, A. et al. The HNRNPA2B1–MST1R–Akt axis contributes to epithelial-to-mesenchymal transition in head and neck cancer. Lab Invest 100, 1589–1601 (2020). https://doi.org/10.1038/s41374-020-0466-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41374-020-0466-8
This article is cited by
-
CSNK1D-mediated phosphorylation of HNRNPA2B1 induces miR-25-3p/miR-93-5p maturation to promote prostate cancer cell proliferation and migration through m6A-dependent manner
Cellular and Molecular Life Sciences (2023)
-
HNRNPA2B1-mediated m6A modification of TLR4 mRNA promotes progression of multiple myeloma
Journal of Translational Medicine (2022)
-
Gene signature of m6A RNA regulators in diagnosis, prognosis, treatment, and immune microenvironment for cervical cancer
Scientific Reports (2022)
-
Heterogeneous nuclear ribonucleoprotein A/B: an emerging group of cancer biomarkers and therapeutic targets
Cell Death Discovery (2022)