Abstract
The etiology of primary Sjögren’s syndrome (pSS) remains unknown, and there is no complete curative drug. In this study, we treated a mouse model of the submandibular gland (SG) protein-immunized experimental Sjögren’s syndrome (ESS) with paeoniflorin-6′-O-benzene sulfonate (termed CP-25) to evaluate the potential therapeutic effects of CP-25. Through in vivo experiments, we found that CP-25 increased saliva flow, alleviated the salivary gland indexes, and improved tissue integrity in the ESS model. The viability of splenocytes and B-lymphocyte migration from spleen were reduced in ESS mice. Furthermore, CP-25 decreased the expression of IgG antibodies, anti-SSA and anti-SSB antibodies and modulated the levels of cytokines in the serum of SS mice. The numbers of total B lymphocytes, plasma cells (PCs), and memory B cells diminished in the salivary gland. Increased expression of the JAK1-STAT1-CXCL13 axis and IFNα was found in human tissue isolated from pSS patients. In vitro, after stimulation with IFNα, the levels of CXCL13 mRNA and CXCL13 in human salivary gland epithelial cells (HSGEC) increased, while CP-25 counteracted the secretion of CXCL13 and downregulated the expression of CXCL13. IFN-α activated the JAK1-STAT1/2-CXCL13 signaling pathway in HSGEC, which was negatively regulated by additional CP-25. As a consequence, B-cell migration was downregulated in coculture with IFN-α-stimulated HSGEC. Taken together, this study demonstrated that the therapeutic effects of CP-25 were associated with the inhibition of the JAK1-STAT1/2-CXCL13 signaling pathway in HSGEC, which impedes the migration of B cells into the salivary gland. We identified the underlying mechanisms of the therapeutic effect of CP-25 and provided an experimental foundation for CP-25 as a potential drug in the treatment of the human autoimmune disorder pSS.
Similar content being viewed by others
Introduction
Primary Sjogren’s syndrome (pSS) is a systemic autoimmune disease, the main pathological feature of which is the infiltration of exocrine glandular lymphocytes. It mainly manifests as dry mouth and dry eyes [1, 2]. The primary mechanism of pSS development is thought to be the damage of the exocrine gland epithelium mediated by an abnormal response of immunocytes to the autoantigens [3, 4].
pSS is an autoimmune epithelitis in which salivary gland epithelial cells (SGECs), also known as immunocytes, play a key pathogenic role [5] by contributing to the recruitment and activation of T and B cells by secreting chemokines and cytokines upon stimulation by viral exposure or IFNα [6]. Glandular inflammatory infiltrates, comprising B cells, T cells, natural killer cells, and other immune cells, along with cytokines, are critical drivers of autoimmunity. Primarily, in addition to producing autoantibodies, B cells are also involved in the synthesis of various proinflammatory cytokines and antigen (Ag) presentation [7]. The pSS is characterized by abnormal pathological B-cell overproduction, such as abnormal distribution of peripheral B-cell subsets, such as abnormal distribution of peripheral B-cell subsets, hyperglobulinemia, etc. [8, 9]. CXCL13 and its receptor CXCR5 are vital for migration, infiltration, and secretion of autoantibodies by B lymphocytes [10]. Some studies have shown that B-cell subsets play a vital role in the immune pathogenesis of pSS, especially memory B cells and plasma cells (PCs) [11, 12].
At present, there is no ideal drug for the specific treatment of pSS. Symptomatic treatment and system therapy are commonly used to relieve the symptoms and delay the development of the disease. However, systemic therapy, including glucocorticoids or immunosuppressive treatment, has adverse reactions, including gastrointestinal ulcers, immunosuppression, and even tumor development [13, 14]. Thus, finding safe and effective therapeutic drugs against pSS is an important research direction. The main active ingredient of total glucosides of peony is paeoniflorin (Pae), which has been used clinically to treat autoimmune diseases [15, 16]. However, Pae is poorly absorbed and takes a long time to exert its effect because of poor permeation. To improve this characteristic, after esterification of Pae, Paeoniflorin-6′-O-benzene sulfonate (termed CP-25) with better fat solubility and bioavailability was obtained [17]. Our research shows that after CP-25 treatment, the lymphocyte infiltration in the salivary glands is reduced, and the salivary flow in NOD/Ltj experimental Sjogren’s syndrome mice (ESS) is also improved [18]. The NOD mouse model has similar symptoms as pSS but lacks its longitudinal stability [19]. At present, many SS-like disease mouse models have been used for exploring SS pathophysiology. Earlier studies have shown that salivary gland inflammation in SMA mice can be induced by repeated immunization of salivary glands with Klebsiella O3 lipopolysaccharide [20]. In addition, after immunizing a mixture of saliva and salivary gland extracts, symptoms of SS-like dry keratoconjunctivitis were also observed, suggesting that saliva-gland-derived proteins may induce SS-like symptoms as autoantigens [21]. The self-Ag that triggers SS in C57BL/6 mice also includes salivary gland proteins [22].
In our study, we successfully built an SS-like disease model in submandibular gland (SG) autoantigen-immunized C57BL/6 mice, which allows us to investigate the ESS physiopathological mechanism in a mouse strain without genetic susceptibility. This model allows us to address the effect of CP-25 on the SG protein-immunized ESS mice and B lymphocytes and to study the mechanism associated with modulating the human submandibular gland epithelial cell (HSGEC) secretion of chemokines, which affects the migration of B-lymphocyte subsets. To this end, SG mice immunized with SG protein were treated with CP-25. The therapeutic effect of CP-25 was observed, and the mechanism related to the regulation of SGECs signaling pathway was analyzed, especially the JAK1-STAT1/2-CXCL13 axis. The study revealed the therapeutic effect and mechanism of CP-25 and provided an experimental basis for its clinical treatment for pSS in the future.
Materials and methods
Animals
C57BL/6 mice (female, 6–7 weeks, 18 ± 2 g) were purchased from the Institute of Biomedical Research, Nanjing University (Certificate No. SCXK(SU)2015-0001). The mice were raised in the SPF laboratory of the Experimental Animal Center of Anhui Medical University. Animal-related experiments were approved by the Ethics Committee for Animal Experimentation of Anhui Medical University (No. 20140186, China).
Reagents and drugs
For details see Table 1.
Induction and treatment of ESS in mice
The ESS model was built using a modified method with SG protein immunization. For the preparation of SG protein, 8-week-old C57BL/6 mice were harvested with bilateral SG, homogenized in PBS, and centrifuged at 12,000 × g for 5 min at 4 °C. The protein concentration of the supernatant was measured by BCA, added to the same volume of Freund’s complete adjuvant and emulsified to 2.5 mg/mL. On day 0, the emulsion was intradermally injected into the tail and back of the 8-week-old mice, and this operation was repeated on day 7. On the 14th day, an equal volume of Freund’s incomplete adjuvant was mixed with SG to form an emulsion of the same concentration to boost immunity and injected in the same way (Fig. 1).
ESS mice were screened for salivary flow in our study on the 6th week after induction. Successfully immunized ESS mice were divided into five groups, at least eight in each group. Another normal group was set up. CP-25 with a concentration of 17.5, 35, or 70 mg/kg and HCQ with a concentration of 80 mg/kg were consecutively administered intragastrically for 2 weeks.
Salivary flow
The mice were anesthetized by intraperitoneal injection of 2.4% pentobarbital sodium at a dose of 5 ml/kg followed by subsequent intraperitoneal injection of 5 mg/kg pilocarpine to induce saliva secretion. After 5 min, a weighed dry cotton ball was placed in the mouse’s mouth to collect saliva, and the cotton ball was weighed after 10 min. The difference in weight of the cotton ball was considered the saliva flow every 10 min.
Indexes for organs
After the mice were sacrificed, the thymus, salivary glands, and spleen were removed under aseptic conditions and weighed. The index of these organs was calculated according to the following formula: organ index (mg/g) = organ weight (mg)/body weight (g).
H&E staining analysis
The mouse SG tissue was collected for H&E staining analysis. The grade of the score was determined according to the severity of lymphocyte infiltration in the tissue. The presence of more than 50 lymphocytes per 4 mm2 was defined as a lymphocytic focus.
The scores were graded as 0 = no lymphocytic infiltration; 1 = slight lymphocytic infiltration; 2 = moderate lymphocytic infiltration but <1 lymphocytic focus; 3 = one lymphocytic focus; and 4 = more than one lymphocytic focus.
Detection of thymocyte and splenocyte viability
After 2 weeks of treatment, the thymus and spleen were sterilely obtained. The lymphocyte separation fluid was used to isolate thymocytes and splenocytes. Then, 50 μl thymocytes (1 × 107/mL) and 50 μl of ConA (10 µg/ml) or 50 μl splenocytes (1 × 107/mL) and 50 μl of 10 µg/ml LPS were added to a 96-well culture plate and incubated for 48 h. Four hours before the end of the culturing, 10 µL of CCK8 was added to each well. The absorbance (A) was measured at 450 nm.
Flow cytometric analysis
Flow cytometry (FCM) detection of splenic lymphocytes and SG lymphocytes was performed. Splenocytes were collected using erythrocyte lysate and a lymphocyte separation fluid. Lymphocytes of the SG were separated with lymphocyte separation fluid. The cell suspension was collected, and antibodies were added to CD19-FITC, PDCA1-APC, and CD27-PE or CD19-FITC and CD4-PE in the polystyrene tube. After incubation at 37 °C in the dark for 40 min, analysis was performed by FCM (Beckman USA) using FlowJo Quest software.
Detection of serum autoantibodies and cytokines
The serum samples were collected from mice. The expression of anti-SSA/Ro, anti-SSB/La, IgG, and CXCL13 in serum was detected by an ELISA kit. IFN-α (10 ng/ml) was used to stimulate HSGECs for 24 and 48 h. The expression of CXCL13 in the cell supernatant was detected by an ELISA kit. The optical density was detected at 450 nm.
The concentrations of IL-2, IL-4, IL-6, IL-17A, TNF-α, and IFN-γ were detected by FCM using CBA mouse cytokine kits.
Human tissue samples
Human labial gland specimens were collected by biopsy from 13 suspected SS patients who were evaluated at the First Affiliated Hospital of Anhui Medical University. According to 2016 ACR/EULAR criteria, 10 pSS patients (all women) were finally diagnosed. Three individuals (all women) who had normal labial biopsy were used as the control group. The patients provided informed consent. The Ethics Committee approved the research at the Anhui Medical University (No.: 20190399).
Immunofluorescence microscopy
For the Leica immunofluorescence microscopy (SP8), frozen sections were incubated with primary anti-mouse or anti-human anti-amylase, anti-CXCL13, and anti-mouse or anti-human JAK1, STAT1, p-JAK1, and p-STAT1. Then, the sections were incubated in the dark with Alexa Fluor 594-conjugated goat anti-rabbit IgG (H + L), and Alexa Fluor 488-conjugated goat anti-mouse IgG (H + L) or Alexa Fluor 647 AffiniPure goat anti-mouse IgG (H + L) fluorescent secondary antibody. ImageJ software was used for the data analysis.
Cell culture
HSGECs and human Maver-1 cells (B-lymphocyte line) were purchased from the European Certified Cell Culture Collection. The HSGECs were cultured in DMEM containing 10% FBS and penicillin/streptomycin (1%) under normal culture conditions, and the human Maver-1 cells were maintained in RPMI-1640 containing 10% FBS.
The HSGECs were stimulated with 10 ng/ml IFN-α with or without tofacitinib (0.1, 1, and 10 μM) for 48 h. Proteins were then extracted from the cells for WB.
B-lymphocyte migration
To determine the migration ability of Maver-1 cells to CXCL13, we used Transwell 24-well plates with 6.5 mm polycarbonate filters. In brief, the HSGECs were stimulated with IFN-α (10 ng/ml) with or without CP-25 (0.1, 1, and 10 μM), tofacitinib (10 μM), and CXCL13-neutralizing antibody (300 ng/ml) placed in the lower part of the well. Next, RPMI-1640 containing 5 × 105 Maver-1 cells was seeded on the inner chamber. After 48 h of incubation, migrating cells were collected for counting by a hemocytometer and protein extraction for WB.
Real-time PCR
IFN-α (10 ng/ml) was used to stimulate the HSGECs for 24 and 48 h. The total RNA of the HSGECs was extracted with TRIzol reagent. The total RNA was reverse transcribed to cDNA by an Advantage cDNA PCR Kit. After quantifying the expression levels of mRNAs for CXCL13 and GAPDH, we maintained HSGECs at 95 °C for 10 min and then performed 40 cycles of 15 s at 95 °C and 60 s at 60 °C. The sequences used were CXCL13: GCTTGAGGTGTAGATGTGTCC and CCCACGGGGCAAGATTTGAA; GAPDH: GGTGAAGGTCGGAGTCAACG and CAAAGTTGTCATGGATGHACC.
Western blot analysis
RIPA lysis buffer was used to lyse HSGEC to obtain protein, including protease inhibitors and phosphatase inhibitors. The quantified protein was mixed with the loading buffer (Beyotime, China) at a ratio of 4:1 and then heated. The samples were electrophoresed by 8% SDS-PAGE and then transferred to a PVDF membrane (Millipore, Bedford, MA, USA). The membrane was blocked in 5% skim milk and then overnight with primary antibodies p-JAK1 (1:500), JAK1, p-STAT1, STAT1, p-STAT2, STAT2, and β-actin (1:1000) at 4 °C. Then, the secondary antibody was added. Protein bands could be detected by chemiluminescence (Pierce, Rockford, IL, USA) and scanned by an ImageQuant LAS 4000 Mini (GE Healthcare Bio-Sciences AB, Uppsala, Sweden). The protein quantification results were normalized to the corresponding β-actin.
Statistical analysis
The results were expressed as the means ± SD. The data analysis used a one-way ANOVA, Mann–Whitney U test, or Dunnett’s multiple comparison test to compare the differences between groups. P < 0.05 was regarded as significant. The calculation was performed using SPSS 16.0. The figures were constructed by GraphPad Prism.
Results
CP-25 can increase saliva flow, reduce the SG index, and improve the histological evaluation of SS mice
After C57BL/6 mice were immunized with SG protein, the disease characteristics of SS appeared. Six weeks after immunization (prior treatment), the saliva flow of the SS model was significantly lower than that of the control group. Following CP-25 (70 mg/kg) treatment for a week, the first positive effects could be detected. After 2 weeks of treatment, CP-25 at a concentration of 35 and 70 mg/kg and HCQ at a concentration of 80 mg/kg significantly increased saliva secretion (Fig. 2a).
C57BL/6 mice were immunized with SG protein, which was emulsified in Freund’s complete adjuvant (injected intradermally on days 0, 7) or Freund’s incomplete adjuvant (injected intradermally on day 14). a CP-25 (35, 70 mg/kg) increased saliva flow in SS mice after a 2-week treatment (\(\overline x\) ± s, n = 8). b CP-25 reduced the salivary gland indexes in SS mice (\(\overline x\) ± s, n = 4–6). c, d CP-25 attenuated lymphocytic infiltration in the salivary gland and significantly improved histological scores in SS mice. #P < 0.05, ##P < 0.01 compared with the control; *P < 0.05, **P < 0.01 compared with the model.
In the untreated control group, the indexes for the thymus, salivary glands, and spleen of the treated groups increased significantly, while treatment with CP-25 at concentrations of 35 and 70 mg/kg reduced the salivary gland. Meanwhile, the spleen indexes were reduced only in the group treated with 70 mg/kg CP-25. There were no effects of CP-25 treatment on the thymus indexes (Fig. 2b).
After 2 weeks of treatment, the histological assessment for the salivary glands showed noticeable differences between the treated and untreated groups. SG protein-immunized mice exhibited tissue damage with lymphocytic infiltration and lymphocytic foci. Compared with the untreated model group, CP-25 at a concentration of 70 mg/kg protected the salivary gland against cell infiltration (Fig. 2c). According to a previously described graduation standard, we assessed the scores based on the lymphocytic foci in every group. CP-25 at a concentration of 70 mg/kg reduced the histological score of the salivary glands (Fig. 2d).
CP-25 inhibits splenocyte viability and regulates the proportion of B-lymphocyte subsets in the spleen of SS mice
Lymphocytes, especially B lymphocytes, are essential for the pathophysiologic mechanism of pSS. Therefore, we confirmed whether the suppression of lymphocyte viability was related to the treatment of CP-25. Lymphocyte viability here means the number of live cells, which further reflects the reactivity of lymphocyte cells. We collected thymocytes and splenocytes from mice after 2 weeks of treatment and analyzed the thymocyte viability (ConA stimulation) and splenocyte viability (LPS stimulation). While there was no significant change in thymocyte viability after ConA-activation in each group, LPS-induced activation of splenocytes significantly increased their viability in ESS model mice compared with the control group. Both CP-25 and HCQ inhibited splenocyte viability after 2 weeks of treatment (Fig. 3a, b).
a The effects of CP-25 on thymocyte viability in SS mice (\(\overline x\) ± s, n = 4–6). b CP-25 inhibited splenocyte viability in SS mice (\(\overline x\) ± s, n = 4). c CD19+CD27+ memory B cells were analyzed by flow cytometry in SS mice. d CP-25 increased memory B cells from splenocytes in SS mice (\(\overline x\) ± s, n = 3–5). e CD19+PDCA1+ plasma B cells were analyzed by flow cytometry in SS mice. f CP-25 decreased plasma B cells from splenocytes in SS mice (\(\overline x\) ± s, n = 3). #P < 0.05, ##P < 0.01 compared with the control; *P < 0.05, **P < 0.01 compared with the model.
Memory B cells and PCs constitute the two vital cellular subpopulations generated in germinal center responses that together facilitate long-term humoral immunity. Therefore, we examined the percentage of defined memory B cell (CD19+CD27+) and PC (CD19+PDCA1+) subsets in the spleen of mice via FCM. The rate of memory B cells was markedly reduced from 4.08% in the untreated group to 2.71% in the model group. After treatment with CP-25 at a concentration of 70 mg/kg and HCQ at a concentration of 80 mg/kg, the proportion of memory B cell subsets can be upregulated (Fig. 3c, d). The PC function was hyperactive in ESS, and the percentage of PCs increased in the untreated model group. The percentage of PC subpopulations decreased after treatment with 70 mg/kg CP-25 and 80 mg/kg HCQ at (Fig. 3e, f).
CP-25 decreases anti-SSA/SSB and IgG antibodies and modulates the level of cytokines from serum in SS mice
CP-25 modulated the percentage of the PC subset. Therefore, we analyzed the effect of CP-25 on the secretory antibodies by PCs. We determined the level of anti-SSA/SSB and IgG antibodies in the serum. These autoantibody levels significantly increased in the SS model group, including anti-SSA/SSB and IgG antibodies. CP-25 and HCQ treatment downregulated the level of anti-SSB, and CP-25 at a concentration of 70 mg/kg reduced the level of anti-SSA and IgG antibody expression (Fig. 4a).
a CP-25 decreased anti-SSA, anti-SSB, and IgG antibodies in SS mice (\(\overline x\) ± s, n = 3–4). b Immune-inflammatory cytokine (IL-2, IL-4, IL-6, IFNγ, TNF-α, and IL-17A) levels were measured using a CBA Mouse Cytokine Kit. c Bar graph illustrating the levels of inflammatory cytokines (\(\overline x\) ± s, n = 3–4). #P < 0.05, ##P < 0.01 compared with the control; *P < 0.05, **P < 0.01 compared with the model.
We further measured the levels of inflammatory cytokines (IL-2, IL-4, IL-6, IL-17A, IFN-γ, and TNF-α) in the serum using the CBA mouse cytokine kit by FCM. Compared with the unimmunized control group, the expression levels of IL-4, IL-6, IL-17A, IFN-γ, and TNF-α in the ESS model group were significantly increased. CP-25 (70 mg/kg) downregulated IL-4 and IL-17A and had significant effects (35 and 70 mg/kg) on IL-6 and TNF-α (17.5, 35, and 70 mg/kg) (Fig. 4b, c).
CP-25 decreases total B lymphocytes, memory B cells, and PCs in the salivary gland
The main tissue affected by lymphocyte infiltration and lymphocytic foci is the salivary glands. Therefore, we analyzed the lymphocyte subsets, including total T and B lymphocytes, memory B cells, and PCs. In the model ESS group, the proportion of total B lymphocytes increased significantly, while treatment with CP-25 at a concentration of 70 mg/kg inhibited the infiltration of B lymphocytes into the glands. There were no obvious abnormalities in total T lymphocytes in the model group salivary glands (Fig. 5a).
a CP-25 decreases total B lymphocytes in the salivary gland. b CP-25 decreases memory B cells in the salivary gland. c CP-25 decreases PCs in the salivary gland (\(\overline x\) ± s, n = 3). The proportion of T and B lymphocytes among total live immune cells. ##P < 0.01 compared with the control; *P < 0.05, **P < 0.01 compared with the model.
B lymphocytes are the main infiltrating cells in ESS model mice salivary glands. We further determined the proportion of CD19+CD27+ memory B cells and CD19+PDCA1+ PCs in the salivary glands. The percentage of PCs and memory B cells was markedly upregulated from an average of 0.508% and 0.49% in the control group to 1.61% and 0.93% in the immunized model group, respectively, and the percentage of PC and memory B cell subsets in the salivary glands was reduced by CP-25 (Fig. 5b, c).
These results suggested that large numbers of B lymphocytes (memory B cells and PCs) migrated to and infiltrated the salivary glands, and CP-25 inhibited the migration and infiltration of B lymphocytes.
HSGEC can secrete the chemokine CXCL13 upon stimulation by IFNα, which is inhibited by CP-25
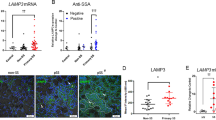
CXCL13 is the essential chemokine that can regulate the migration and infiltration of B lymphocytes into inflammatory glands of pSS. First, we detected the expression of CXCL13 in the serum of ESS mice by CP-25. CXCL13 expression increased in ESS mice, while CP-25 decreased CXCL13 levels (Fig. 6a). We further determined the expression of CXCL13 in the SGECs (labeled by α-amylase) of mouse salivary glands by immunofluorescence. The results indicated that the expression of CXCL13 on SGECs markedly increased in the ESS group. CP-25 decreased the CXCL13 levels in salivary gland SGECs (Fig. 6b).
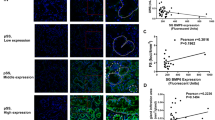
Next, we analyzed the expression of CXCL13 and IFNα in human labial gland specimens. There were significant lymphocytic infiltrations and lymphocytic foci in the glands from pSS patients. The expression of CXCL13 in SGEC (labeled by α-amylase) and IFNα was markedly increased in pSS patients (Fig. 7a–c).
a Histopathological HE is the staining of human labial gland specimens from pSS patients. b The expression of CXCL13 (green) in SGEC(red) of human labial gland specimens from pSS patients by immunofluorescence. c The expression of IFNa in SGEC of human labial gland specimens from pSS patients by immunohistochemical. d The effect of IFNa (10 ng/ml) on CXCL13 mRNA by RT-PCR and CXCL13 in SGECs by ELISA for 24 and 48 h (\(\overline x\) ± s, n = 3). *P < 0.05, **P < 0.01 compared with the control. e CP-25 reduces the expression of CXCL13 (red) in SGEC stimulated by IFNa for 48 h in vitro by immunofluorescence. The nucleus is labeled by DAPI (blue).The nucleus is labeled by DAPI (blue).
Furthermore, we cultured HSGEC and stimulated the cells with IFNα. We detected the CXCL13 mRNA by qPCR and the CXCL13 level by ELISA. The CXCL13 mRNA and CXCL13 levels were significantly increased in HSGECs stimulated by IFNα and inhibited by CP-25 (10−5 M) (Fig. 7d, e).
IFNα activated the JAK1-STAT1/2-CXCL13 signaling pathway in HSGECs, and CP-25 can inhibit the signaling pathway and reduce the secretion of CXCL13
HSGEC can secrete chemokines CXCL13 upon stimulation by IFNα. We explored which signaling pathways are involved in secreting chemokines CXCL13. To this end, we analyzed the activation of the JAK1-STAT1 signaling pathway in the labial gland specimens of pSS patients by immunofluorescence (Fig. 8a).
a The effect of the JAK inhibitor, tofacitinib, on the JAK1-STAT1/2 signaling pathway in SGEC stimulated by IFNa. SGECs were treated with IFNa (10 ng/ml) in the presence or absence of tofacitinib (0.1, 1, and 10 μM) or SHR0302 (0.1, 1, and 10 μM) for 48 h (\(\overline x\) ± s, n = 3), *P < 0.05, **P < 0.01 compared with the control group. #P < 0.05, ##P < 0.01 compared with the IFNa group. b Activation of the JAK1-STAT1 signaling pathway in human labial gland specimens by immunofluorescence. c The effect of CP-25, the JAK inhibitor, and the CXCL13 inhibitor (300 ng/ml CXCL13-neutralizing antibody) on Maver-1 cell (b lymphocyte line) migration (\(\overline x\) ± s, n = 3), **P < 0.01 compared with the control group. #P < 0.05, ##P < 0.01 compared with the IFNa group. d The effect of CP-25 on the JAK1-STAT1/2 signaling pathway in SGEC stimulated by IFNa. SGEC were treated with IFNa (10 ng/ml) in the presence or absence of CP-25 (0.1, 1, and 10 μM) (\(\overline x\) ± s, n = 3). **P < 0.01 compared with the control group. #P < 0.05 compared with the IFNa group.
Furthermore, we determined the signaling of JAK1-STAT1/2 in vitro. The pathway was activated in HSGECs stimulated by IFNα. Tofacitinib, as an inhibitor of JAK, can significantly inhibit the activation of the JAK1-STAT1/2 signaling pathway stimulated by IFNα (Fig. 8b). The results were similar to the labial gland specimens of pSS patients.
To observe the effect of CP-25, the JAK inhibitor, and the CXCL13 inhibitor on B-cell migration, we cocultivated a human B-lymphocyte line (Maver-1) and HSGECs activated by IFNα together with inhibitors and determined the migration of B cells. B-cell migration significantly increased in the coculture with SGECs stimulated by IFNα. Both the JAK inhibitor and the CXCL13 inhibitor could inhibit B-cell migration. The results proved that IFNα activated the JAK1-STAT1/2-CXCL13 signaling pathway in SGEC, promoting B-cell migration (Fig. 8c). CP-25 could inhibit B-cell migration in the coculture with HSGECs stimulated by IFNα. In addition, we detected that CP-25 (10−5 M) significantly inhibited the activation of the JAK1-STAT1/2 signaling pathway stimulated by IFNα (Fig. 8d).
Discussion
pSS is a systemic autoimmune disease, and its clinical manifestations are mainly dry mouth and dry eyes. In the present study, we successfully implemented an SS-like disease model.
Saliva flow and salivary gland biopsy are valuable tools in pSS diagnostics. The lymphocytic focus score plays an important role in pSS classification criteria as a routine clinical diagnostic [23]. Extrinsic triggers are also important factors involved in SS pathogenesis. Immunization with specific autoantigens can induce the breakdown of immune-tolerance and result in glandular inflammation and dysfunction, resembling the symptoms of SS patients. Currently, SS-inducing Ags include Ro peptide, M3R peptide, carbonic anhydrase 2 (CA2), and SG protein extract [24]. Most models use the clinical manifestations of SS patients to produce autoantibodies against Ro, M3R, and CA2 peptides as indicators; meanwhile, information on the progress or onset of pSS is limited, and pSS requires a long time to be established in a mouse model. In our study, after immunization with SG protein, C57BL/6 mice developed pSS symptoms, such as decreased saliva volume, damaged SG tissue with lymphocytic infiltration, and increased autoantibody levels in serum. Treatment with CP-25 increased saliva secretion, attenuated lymphocytic infiltration, and reduced histological scores according to the lymphocytic foci. These results indicate that CP-25 could relieve the symptoms of ESS.
Anti-SSA/SSB antibodies are signs of pSS disease [25]. However, the exact role of these autoantibodies in the pathogenesis is unclear. The absence of anti-SSA/SSB in pSS is associated with a lower prevalence of B cells [26]. In our mouse model CP-25 decreased anti-SSA/SSB and IgG antibodies, which can be associated with modulating B cells. B-cell overactivity and lymph node hyperplasia are unique features of pSS; thus, B cells play a vital role in the pathogenesis of this disease [27]. In addition, we detected the proinflammatory cytokines in serum, including IL-2, IL-4, IL-6, IL-17A, IFNγ, and TNF-α, which can disturb the secretory function of salivary glands and affect the function of B cells. These proinflammatory cytokines were increased in ESS, and CP-25 reduced the secretion of these cytokines. In this study, LPS-induced splenocyte viability was upregulated in the ESS model, and CP-25 inhibited splenocyte viability after 2 weeks of treatment. This indicates that CP-25 modulates autoantibody secretion and the cell viability of B cells.
The germinal center can produce “classic” memory B cells, express surface CD27, and undergo isotype switch [28]. When memory B cells encounter lower levels of immune Ag again, they can quickly differentiate into high-affinity PCs [29]. Memory B cells (CD19+ and CD27+) secrete a large number of proinflammatory cytokines related to the pathogenic factors that occur during autoimmunity [30, 31]. We detected the proportion of memory B cells and PCs from the spleen, and the results showed that CD27+ memory B cells were markedly reduced while PCs increased, which caused an increase in autoantibodies. The reason for the lower CD27+ memory B cells is still elusive. The lower proportion of memory B cells was related to the length and severity of the disease, which indicates that memory B cells may be potential prognostic markers [10]. Our results share similarities with previous publications [32], which described the decrease in memory B cells in pSS. In this work, CD27+ memory B cells in the peripheral blood of patients with pSS were significantly reduced, while memory B cells accumulated in inflamed SG [33, 34]. The accumulation of memory B cells in the SG of SS patients reduces the proportion of peripheral blood cells [35]. We further detected memory B cells and PCs from mice salivary glands. The results were similar to a previous study that showed that the percentage of memory B cells was increased in the SG of ESS mice. These results suggested that memory B cells migrate slowly to salivary glands in pSS. CP-25 could downregulate the proportion of memory B cells in the SG by modulating the migration of B cells.
Dysregulated interactions of B cells with CXCL13 may be closely related to the inflammatory immune response, the progress of ectopic germinal center structures [36, 37] and peripheral memory B cell irregularities. CXCL13 binding to CXCR5, a specific chemoattractant for B cell, is an important response factor for B-cell migration into the inflammatory site. CXCL13 plays a key role in lymphoid organ formation. Indeed, lymph node organogenesis was partially blocked in transgenic mice lacking CXCL13 and its receptor CXCR5 [38]. Abnormal SGEC-generated CXCL13 mediates progressive B-cell aggregation in salivary glands [39]. In a tissue biopsy of pSS patients, stimulation by virus or IFNα could induce SGECs to secrete some cytokines in vitro, such as BAFF, IL-6, and CXCL13 [40,41,42]. IFNα was strongly expressed in the peripheral blood of pSS patients, which was related to high disease activity, the presence of autoantibodies and hypergammaglobulinemia [43]. Our results confirmed the increased expression of CXCL13 and increased IFNα in human labial gland specimens. IFNα can stimulate HSGECs to produce the chemoattractant CXCL13 in vitro. A CXCL13 inhibitor (CXCL13-neutralizing antibody) can inhibit B-lymphocyte migration, demonstrating the role of CXCL13 in B-lymphocyte migration. CP-25 has a similar effect and inhibits B-cell migration. Furthermore, high expression of CXCL13 promotes migration of B cells into the salivary gland, which explains why memory B cells decrease in peripheral blood and spleen and increase in salivary glands.
Viral RNA, DNA, or protein initiate the production of IFNs, which activate downstream signaling cascades, exerting their physiological effects, and the JAK/STAT signaling cascade is the key pathway of IFNα [44]. IFNα increases JAK kinase activity, thereby phosphorylating tyrosine residues [45]. The formation of the binding sites of STAT1 and STAT2 is promoted by this phosphorylation, which is subsequently phosphorylated by JAK. Tofacitinib can be used as a JAK inhibitor [46]. We found that the JAK1-STAT1 signaling pathway was activated in the human labial gland specimens. Our results further demonstrated that IFNα activated the JAK1-STAT1/2 signaling pathway in HSGEC, and the JAK inhibitor tofacitinib inhibited the activation of the JAK1-STAT1/2 signaling pathway in HSGECs stimulated by IFNα and thus inhibited the migration of B lymphocytes. A CXCL13 inhibitor has similar effects and inhibits B-lymphocyte migration. The results demonstrated that IFNα-activated JAK1-STAT1/2-CXCL13 signaling promoted the migration of B lymphocytes. CP-25 inhibited JAK1-STAT1/2-CXCL13 signaling and reduced the migration of B lymphocyte.
In summary, we successfully established the SS model in C57BL/6 mice using SG protein and confirmed that CP-25 treatment increased saliva secretion, attenuated lymphocytic infiltration, and reduced the histopathology scores of salivary glands. Furthermore, CP-25 inhibits the autoantibody secretion and cell viability of inflammatory B cells in the salivary glands and reduces its memory B cells and PCs. The therapeutic effects of CP-25 probably are associated with inhibiting the JAK1-STAT1/2-CXCL13 signaling pathway in SGECs, and this signaling pathway is related to the migration of B cells to salivary glands. The study identified the therapeutic effects of CP-25 and provided the experimental foundation for CP-25 to become a potential drug for the treatment of pSS.
References
Sun X, Lu L, Li Y, Yang R, Shan L, Wang Y. Increased risk of thyroid disease in patients with Sjogren’s syndrome: a systematic review and meta-analysis. Peer J. 2019;7:e6737.
Bodewes ILA, van der Spek PJ, Leon LG, Wijkhuijs AJM, van Helden-Meeuwsen CG, Tas L, et al. Fatigue in Sjögren’s syndrome: a search for biomarkers and treatment targets. Front Immunol. 2019;10:312.
Kamiński B. Laryngological manifestations of Sjögren’s syndrome. Reumatologia. 2019;57:37–44.
Xian Z, Fu D, Liu S, Yao Y, Gao C. Association between B cell growth factors and primary Sjögren’s syndrome-related autoantibodies in patients with non-Hodgkin’s lymphoma. J Immunol Res. 2019;2019:7627384.
Barrera MJ, Bahamondes V, Sepúlveda D, Quest AF, Castro I, Cortés J, et al. Sjögren’s syndrome and the epithelial target: a comprehensive review. J Autoimmun. 2013;42:7–18.
Ambrosi A, Wahren-Herlenius M. Update on the immunobiology of Sjögren’s syndrome. Curr Opin Rheumatol. 2015;27:468–75.
Mielle J, Tison A, Cornec D, Le Pottier L, Daien C, Pers JO. B cells in Sjögren’s syndrome: from pathophysiology to therapeutic target. Rheumatology. 2019. https://doi.org/10.1093/rheumatology/key332.
James K, Chipeta C, Parker A, Harding S, Cockell SJ, Gillespie CS, et al. B-cell activity markers are associated with different disease activity domains in primary Sjögren’s syndrome. Rheumatology. 2018;57:1222–7.
Hansen A, Lipsky PE, Dörner T. B-cell lymphoproliferation in chronic inflammatory rheumatic diseases. Nat Clin Pract Rheumatol. 2007;3:561–9.
Armas-González E, Domínguez-Luis MJ, Díaz-Martín A, Arce-Franco M, Castro-Hernández J, Danelon G, et al. Role of CXCL13 and CCL20 in the recruitment of B cells to inflammatory foci in chronic arthritis. Arthritis Res Ther. 2018;20:114.
Barcelos F, Martins C, Papoila A, Geraldes C, Cardigos J, Nunes G, et al. Association between memory B-cells and clinical and immunological features of primary Sjögren’s syndrome and Sicca patients. Rheumatol Int. 2018;38:1063–73.
Bird AK, Meednu N, Anolik JH. New insights into B cell biology in systemic lupus erythematosus and Sjögren’s syndrome. Curr Opin Rheumatol. 2015;27:461–7.
Fox RI, Fox CM, Gottenberg JE, Dörner T. Treatment of Sjögren’s syndrome: current therapy and future directions. Rheumatology. 2019.https://doi.org/10.1093/rheumatology/kez142.
Chen X, Wu H, Wei W. Advances in the diagnosis and treatment of Sjogren’s syndrome. Clin Rheumatol. 2018;37:1743–9.
Luo J, Jin DE, Yang GY, Zhang YZ, Wang JM, Kong WP, et al. Total glucosides of paeony for rheumatoid arthritis: a systematic review of randomized controlled trials. Complement Ther Med. 2017;34:46–56.
Jin L, Li C, Li Y, Wu B. Clinical efficacy and safety of total glucosides of paeony for primary Sjögren’s syndrome: a systematic review. Evid Based Complement Alternat Med. 2017;2017:3242301.
Wang C, Yuan J, Zhang LL, Wei W. Pharmacokinetic comparisons of paeoniflorin and paeoniflorin-6’O-benzene sulfonate in rats via different routes of administration. Xenobiotica. 2016;46:1142–50.
Gu F, Xu S, Zhang P, Chen X, Wu Y, Wang C, et al. CP-25 alleviates experimental Sjögren’s syndrome features in NOD/Ltj mice and modulates T lymphocyte subsets. Basic Clin Pharmacol Toxicol. 2018. https://doi.org/10.1111/bcpt.13025.
Lodde BM, Mineshiba F, Kok MR, Wang J, Zheng C, Schmidt M, et al. NOD mouse model for Sjögren’s syndrome: lack of longitudinal stability. Oral Dis. 2006;12:566–72.
Mu MM, Chakravortty D, Takahashi K, Kato Y, Sugiyama T, Koide N, et al. Production of experimental autoimmune sialadenitis in mice immunized with homologous salivary gland extract and Klebsiella O3 lipopolysaccharide. J Autoimmun. 2001;16:29–36.
Jiang G, Ke Y, Sun D, Li H, Ihnen M, Jumblatt MM, et al. A new model of experimental autoimmune keratoconjunctivitis sicca (KCS) induced in Lewis rat by the autoantigen Klk1b22. Invest Ophthalmol Vis Sci. 2009;50:2245–54.
Lin X, Rui K, Deng J, Tian J, Wang X, Wang S, et al. Th17 cells play a critical role in the development of experimental Sjögren’s syndrome. Ann Rheum Dis. 2015;74:1302–10.
Blokland SLM, Hillen MR, van Vliet-Moret FM, Bikker A, de Jager W, Pandit A, et al. Salivary gland secretome: a novel tool towards molecular stratification of patients with primary Sjögren’s syndrome and non-autoimmune sicca. RMD Open. 2019;5:e000772.
Peck AB. What can Sjögren’s syndrome-like disease in mice contribute to human Sjögren’s syndrome? Clin Immunol. 2017;182:14–23.
Nair JJ, Singh TP. Sjogren’s syndrome: review of the aetiology, pathophysiology & potential therapeutic interventions. J Clin Exp Dent. 2017;9:e584–9.
Quartuccio L, Baldini C, Bartoloni E, Priori R, Carubbi F, Corazza L, et al. Anti-SSA/ SSB-negative Sjögren’s syndrome shows a lower prevalence of lymphoproliferative manifestations, and a lower risk of. Autoimmun Rev. 2015;14:1019–22.
Hansen A, Daridon C, Dörner T. What do we know about memory B cells in primary Sjögren’s syndrome? Autoimmun Rev. 2010;9:600–3.
Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60.
Jelinek DF, Splawski JB, Lipsky PE. Human peripheral blood B lymphocyte subpopulations: functional and phenotypic analysis of surface IgD positive and negative subsets. J Immunol. 1986;136:83–92.
Sanz I, Lee FE. B cells as therapeutic targets in SLE. Nat Rev Rheumatol. 2010;6:326–37.
Quan C, Yu H, Qiao J, Xiao B, Zhao G, Wu Z, et al. Impaired regulatory function and enhanced intrathecal activation of B cells in neuromyelitis optica: distinct from multiple sclerosis. Mult Scler. 2013;19:289–98.
Roberts ME, Kaminski D, Jenks SA, Maguire C, Ching K, Burbelo PD, et al. Primary Sjögren’s syndrome is characterized by distinct phenotypic and transcriptional profiles of IgD+ unswitched memory cells. Arthritis Rheumatol. 2014;66:2558–69.
Hansen A, Odendahl M, Reiter K, Jacobi AM, Feist E, Scholze J, et al. Diminished peripheral blood memory B cells and accumulation of memory B cells in the salivary glands of patients with Sjögren’s syndrome. Arthritis Rheum. 2002;46:2160–71.
Hansen A, Jacobi A, Pruss A, Kaufmann O, Scholze J, Lipsky PE, et al. Comparison of immunoglobulin heavy chain rearrangements between peripheral and glandular B Cells in a patient with primary Sjögren’s syndrome. Scand J Immunol. 2003;57:470–9.
Aqrawi LA, Skarstein K, Øijordsbakken G, Brokstad KA. Ro52-and Ro60-specific B cell pattern in the salivary glands of patients with primary Sjögren’s syndrome. Clin Exp Immunol. 2013;172:228–37.
Bombardieri M, Pitzalis C. Ectopic lymphoid neogenesis and lymphoid chemokines in Sjogren’s syndrome: at the Interplay between chronic inflammation, autoimmunity and lymphomagenesis. Curr Pharm Biotechnol. 2012;13:1989–96.
Kramer JM, Klimatcheva E, Rothstein TL. CXCL13 is elevated in Sjögren’s syndrome in mice and humans and is implicated in disease pathogenesis. J Leukoc Biol. 2013;94:1079–89.
Mebius RE. Organogenesis of lymphoid tissues. Nat Rev Immunol. 2003;3:292–303.
Campos J, Hillen MR, Barone F. Salivary gland pathology in Sjögren’s syndrome. Rheum Dis Clin North Am. 2016;42:473–83.
Nakamura H, Takahashi Y, Yamamoto-Fukuda T, Horai Y, Nakashima Y, Arima K, et al. Direct infection of primary salivary gland epithelial cells by human T lymphotropic virus type I in patients with Sjögren’s syndrome. Arthritis Rheumatol. 2015;67:1096–106.
Ittah M, Miceli-Richard C, Gottenberg JE, Sellam J, Eid P, Lebon P, et al. Viruses induce high expression of BAFF by salivary gland epithelial cells through TLR- and type-I IFN-dependent and independent pathways. Eur J Immunol. 2008;38:1058–64.
Barone F, Bombardieri M, Rosado MM, Morgan PR, Challacombe SJ, De Vita S, et al. CXCL13, CCL21, and CXCL12 expression in salivary glands of patients with Sjogren’s syndrome and MALT lymphoma: association with reactive and malignant areas of lymphoid organization. J Immunol. 2008;180:5130–40.
Brkic Z, Maria NI, van Helden-Meeuwsen CG, van de Merwe JP, van Daele PL, Dalm VA, et al. Prevalence of interferon type I signature in CD14 monocytes of patients with Sjögren’s syndrome and association with disease activity and BAFF gene expression. Ann Rheum Dis. 2013;72:728–35.
Raftery N, Stevenson NJ. Advances in anti-viral immune defence: revealing the importance of the IFN JAK/STAT pathway. Cell Mol Life Sci. 2017;74:2525–35.
Brooks AJ, Dai W, O’Mara ML, Abankwa D, Chhabra Y, Pelekanos RA, et al. Mechanism of activation of protein kinase JAK2 by the growth hormone receptor. Science. 2014;344:1249783.
Wu H, Yan S, Chen J, Luo X, Li P, Jia X, et al. JAK1-STAT3 blockade by JAK inhibitor SHR0302 attenuates inflammatory responses of adjuvant-induced arthritis rats and decreases Th17 and total B cells. Joint Bone Spine. 2016;83:525–32.
Funding
This study was supported by the National Nature Science Foundation of China (Nos 81673444 and 81302784), Provincial Natural Science Research Project of Anhui Province (Nos KJ2017A182 and KJ2018A0166), and Key Projects of Anhui Province University Outstanding Youth Talent Fund (gxyqZD2018023).
Author information
Authors and Affiliations
Contributions
WW designed the study and acted as a research coordinator. HK edited the paper. HW designed the study, conducted the experiments, collected and analyzed the data, and wrote the paper. XC and FG conducted the experiments and collected and analyzed the data. SX, PZ, QL, QZ, XW, and CW helped to conduct the experiments. The final paper has been read and approved by all authors.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, H., Chen, X., Gu, F. et al. CP-25 alleviates antigen-induced experimental Sjögren’s syndrome in mice by inhibiting JAK1-STAT1/2-CXCL13 signaling and interfering with B-cell migration. Lab Invest 101, 1084–1097 (2021). https://doi.org/10.1038/s41374-020-0453-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41374-020-0453-0