Abstract
The abnormal differentiation of T helper 17 (Th17) cells is considered a vital promoter of immune thrombocytopenia (ITP) progression. Therefore, this study investigated the role of miR-199a-5p in Th17 differentiation and determined whether extracellular vesicles (EVs) derived from miR-199a-5p-modified adipose-derived mesenchymal stem cells (ADSCs) could relieve ITP by inhibiting Th17 differentiation. The miR-199a-5p level was lessened in the spleen tissues of mice with ITP, while the signal transducer and activator of transcription 3 (STAT3) expression and the population of Th17 in CD4+T cells were boosted. Functionally, miR-199a-5p overexpression lowered IL-17 secretion and the proportion of Th17/CD4+T cells. Further investigation showed that miR-199a-5p directly targeted STAT3 mRNA, and negatively modulated its expression. STAT3 overexpression was found to facilitate Th17 differentiation, which was subsequently abolished by miR-199a-5p overexpression. EVs isolated from miR-199a-5p-modified ADSCs (miR-199a-5p-EVs) highly expressed miR-199a-5p and could restrain CD4+T cells polarized toward a Th17 phenotype in vitro. Administering of miR-199a-5p-EVs elevated platelet counts and decreased the proportion of Th17/CD4+T cells in mice with ITP. Taken together, EVs derived from miR-199a-5p-modified ADSCs vividly repressed Th17 differentiation by transferring miR-199a-5p to CD4+T cells, thus ameliorating experimental ITP.
Similar content being viewed by others
Introduction
Immune thrombocytopenia (ITP) is an acquired autoimmune disease, characterized by increasing platelet (PLT) destruction and decreasing PLT production [1]. In recent years, ITP development is considered to be attributed to a humoral immunity disorder [2]. However, emerging evidence reveals that dysfunctional cellular immunity also contributes to ITP development [3].
T helper 17 (Th17) cells, a subset of CD4+ Th, are proposed to be involved in the development of autoimmune disorders by producing IL-17, IL-6, and other pro-inflammatory mediators [4]. To date, various researchers have observed apparent Th17 elevation in ITP patients. Zhu et al. [5] revealed that the CD3+CD8−IL17+ Th17 cells were more abundant in their sampled ITP patients’ plasma than in that of healthy volunteers. Qiao et al. [6] also showed that relative to the peripheral blood mononuclear cells (PBMCs) of recovering ITP patients, the PBMCs of patients with active ITP presented superior levels of Th17 and IL-17. The repression of Th17 cell differentiation has also been shown to raise blood PLT counts in a mouse ITP model [7]. These data suggest that abnormal Th17 differentiation is a vital promoter of ITP progression, and inhibiting Th17 differentiation is a promising therapeutic approach.
A genome-wide expression analysis conducted by Jernås et al. [8] revealed the dysregulation of microRNAs (miRNAs) in ITP patients, suggesting that miRNAs participate in ITP progression. Garabet et al. [9] have also screened out 81 differentially expressed miRNAs in ITP patients using a miRNA PCR panel. Of these, the miR-199a-5p level was lower in ITP patients but higher after treatment, hinting that miR-199a-5p plays a potential role in ITP. As has been previously reported, miRNAs reduce gene expression by binding to the mRNAs of target genes [10]. In our previous experiments, a bioinformatics database, TargetScan, forecasted that miR-199a-5p could target the mRNA of the signal transducer and activator of transcription 3 (STAT3), a key transcription factor that promotes Th17 cell differentiation [11]. This indicates that miR-199a-5p may suppress CD4+T cells polarized toward a Th17 phenotype by inhibiting STAT3 expression.
In recent years, due to the strong immunosuppressive activity of mesenchymal stem cells (MSCs), their potential therapeutic effects on autoimmune diseases have aroused widespread research interest [12]. Several studies have proven that adipose-derived mesenchymal stem cells (ADSCs), a type of MSCs isolated from fat tissues [13], could alleviate experimental arthritis, experimental autoimmune diabetes, etc. [14, 15], indicating the potential applications of ADSCs for treating autoimmune diseases. As has been previously reported, MSCs exert their biological functions by releasing paracrine factors [16]. Extracellular vesicles (EVs), as paracrine factors secreted by ADSCs, are deemed immunomodulatory mediators in the differentiation and activation of various T-cell subsets [17]. In addition, as small extracellular membrane vesicles containing nucleic acids (such as miRNAs), EVs could exert targeted regulations on genes through function as biological delivery vehicles for miRNA transfer. Qu et al. [18] verified that EVs released from miR-181-5p-modified ADSCs activated autophagy in hepatic stellate cells by targeting STAT3 and B-cell lymphoma-2, thus significantly relieving CCl4-induced liver fibrosis in mice. However, it remains unknown whether EVs derived from miRNA-modified ADSCs could regulate abnormal Th17 differentiation in ITP by transferring miRNAs.
Inspired by previous studies, the present research speculated that, in ITP, the low expression of miR-199a-5p contributes to the abnormal differentiation of Th17 cells, and EVs derived from miR-199a-5p-modified ADSCs could slow down ITP progression by enhancing miR-199a-5p expression.
Materials and methods
ITP mouse model and EV treatment
Male BALB/c mice (3 months old) were provided by the Shanghai SLAC Laboratory Animal Co., Ltd (China). Mice were stochastically assigned to the ITP group and Control group (n = 8/group). In the ITP group, mice received intraperitoneal injections of MWReg30 (CD41 monoclonal antibody, ThermoFisher, USA) at a gradually increased dose (Days 0 and 1: 68 μg/kg; Day 2: 102 μg/kg; and Days 3–8: 136 μg/kg). Mice in the Control group underwent equal volumes of PBS injections.
To examine the role of miR-199a-5p-EVs in vivo, the ITP group was randomly assigned to receive miR-199a-5p-EVs (EVs derived from ADSCs transfected with miR-199a-5p agomir) (n = 8) and NC-EVs (EVs derived from ADSCs transfected with the negative control of miR-199a-5p agomir) (n = 8). On Day 0, the mice in both groups received the intraperitoneal injection of MWReg30 (68 μg/kg). On Day 1, except the intraperitoneal injection of MWReg30 (68 μg/kg), the mice also received tail intravenous injections of miR-199a-5p-EVs (0.5 μg/μl, 100 μl) or NC-EVs (0.5 μg/μl, 100 μl), respectively. The mice in the ITP group were modeled on Days 2–8 according to the above methods.
On Days 0–8, blood was collected from the mice’s caudal veins, and PLT numbers in the blood samples were examined via a Beckman-coulter LH750 automatic blood cell counter (USA). All mice were sacrificed on Day 8 and the spleen tissues and whole blood were collected from each mouse. The Ethics Committee of The Children’s Hospital of Soochow University approved the protocol for these animal experiments.
Isolation of naive CD4+T cells and induction of Th17 differentiation in vitro
The EasySep™ Mouse Naive CD4+T cell Isolation Kit (STEMCELL, Canada) was employed to extract the naive CD4+T cells from the spleen tissues of normal mice. The extracted cells were then purified to >95% via the FACSVerse™ flow cytometer (BD, Germany) with anti-mouse CD4 FITC (Abcam, UK). The naive CD4+T cells were cultured for 4 days in an RPMI 1640 medium containing 15% FBS, 5 μg/ml plate-bound anti-CD3, and 1 μg/ml soluble anti-CD28 antibodies.
For Th17 cell differentiation, the CD4+T cells were incubated with TGF-β (5 ng/ml), IL-6 (100 ng/ml), anti-IFN-γ (10 mg/ml), anti-IL-4 (10 mg/ml), and IL-23 (50 ng/ml) for 96 h as previously described [19].
Flow cytometry analysis
Spleen tissues were ground into a homogenate, filtered, and centrifuged. The centrifugal precipitate was resuspended in PBS, followed by staining with anti-mouse CD4 FITC (Abcam, UK) for 10 min in the dark. Anti-mouse IL-17 PE (eBioscience, USA) was added to the cell suspension, and the population of Th17 in CD4+T cells was determined using a flow cytometer. For cell samples, the collected cells were diluted to 1 × 106 cells/ml using PBS, and the ratio of Th17 in CD4+T cells was determined via the method described above.
ELISA
To measure the level of IL-17, the serum of mice and the supernatant of cells were collected. Then, the IL-17 level was determined using a mouse IL-17 ELISA kit (BioLegend, USA).
Cell transfection
miR-199a-5p agomir/antagomir and their negative control (agomir NC and antagomir NC) were obtained from RIBOBIO (China). For agomir/antagomir transfection, CD4+T cells or ADSCs were incubated with Opti-MEM containing agomir/antagomir/negative controls. Six hours later, 1 ml RPMI 1640 complete medium was added to the wells. Forty-two hours later, cells were harvested for subsequent experiments.
PMIG-STAT3 and its negative control (pMIG) were also obtained from RIBOBIO (China). For retroviral vector infection, CD4+T cells were infected with the RPMI 1640 medium containing pMIG-STAT3 or pMIG and 10 µg/ml polybrene for 90 min.
qRT-PCR
TRIZOL reagent was used to isolate the total RNA samples from cells and spleen tissues of mice. A Total Exosome RNA & Protein Isolation Kit (ThermoFisher, USA) was employed to isolate the RNA samples from EVs. After examining the concentration and purity of RNA, the eligible RNA samples were reverse transcribed into cDNA. Then, miR-199a-5p expression was measured using TaqMan® Non-coding RNA Assays (Thermo Fischer Scientific, USA). U6 was used as an endogenous control.
Dual-luciferase reporter assay
The binding sites between miR-199a-5p and STAT3 were predicted by Targetscan. Isolated naive CD4+T cells were stimulated with anti-CD3 and anti-CD28 for 4 days. Then, the activated CD4+T cells were cotransfected with STAT3-wild-type (WT) 3′UTR luciferase reporter/STAT3-mutant-type (MUT) 3′UTR luciferase reporter and miR-199a-5p agomir/agomir NC. Luciferase activities were measured 2 days after transfection.
Western blot
Western blot was conducted as previously described [20]. The primary antibodies used in this study were as follows: anti-STAT3 (1:2000; Abcam, UK), anti-p-STAT3 (1:5000, Abcam), anti-retinoic acid receptor-related orphan receptor-gamma-t (RORγt; 1:250; eBioscience), anti-CD63 (1:1000; Abcam), anti-CD81 (1:20000; Abcam), and anti-Calnexin (1:5000; Abcam).
Isolation and identification of ADSCs
ADSCs were isolated from mice as previously described [21]. The differentiation ability of ADSCs was examined using ADSCs in passage 3. For adipogenic differentiation, ADSCs were maintained in an adipogenic medium for 14 days. Then, the adipogenic differentiation of ADSCs was measured via Oil Red O staining. For osteogenic differentiation, ADSCs were cultured in an osteogenic medium for 21 days. Then, the osteogenic differentiation of ADSCs was measured via Alizarin Red S staining. The components of the adipogenic/osteogenic media were the same as the previous study [18].
Isolation of EVs
After 48 h of transfection, EVs were isolated from the supernatant of miRNA-modified ADSCs utilizing Exoquick-TC (System Biosciences, Canada). EVs were then analyzed by western blot and visualized using a transmission electron microscope.
Statistical analysis
Data were presented as mean ± standard deviation and analyzed by SPSS 18.0 software. Data from the two experimental groups were analyzed through the Student’s t test. P value < 0.05 was deemed statistically significant.
Results
The ITP mouse model presented a higher proportion of Th17/CD4+T cells and a lower miR-199a-5p expression
As shown in Fig. 1A, the PLT counts were lower in mice from the ITP group than the Control group from the 1st day after injection, confirming that ITP was successfully established in the mouse model. The results of Fig. 1B–D showed that the serum level of IL-17 and the population of Th17 cells in splenic CD4+T cells of the ITP group were higher than in the Control group. Besides, a decline in splenic miR-199a-5p level (Fig. 1E) and elevations in splenic STAT3 and pSTAT3 protein levels (Fig. 1F, G) were observed in ITP mice.
BALB/c mice were divided into the ITP group (n = 8) and the Control group (n = 8). A Blood platelet (PLT) counts were performed using an automatic blood cell counter on Days 0–8. ***P < 0.001. B The serum level of IL-17 was detected using ELISA. The percentage of Th17 cells was measured in splenic CD4+T cells from mice using flow cytometry. Representative histograms were shown in (C) and the quantitative result was shown in (D). **P < 0.01. E miR-199a-5p expression was detected in spleen tissues of mice by qRT-PCR. The protein levels of signal transducer and activator of transcription 3 (STAT3) and phosphorylated-STAT3 (pSTAT3) were detected in spleen tissues of mice using western blot. The quantification of band densitometry was shown in (F) and the representative bands were shown in (G). ***P < 0.001 vs. Control.
miR-199a-5p overexpression restrained Th17 cell differentiation
The influence of miR-199a-5p on Th17 cell differentiation was subsequently explored. CD4+T cells were transfected with miR-199a-5p agomir/agomir NC, followed by the induction of Th17 differentiation. The miR-199a-5p expression (Fig. 2A) confirmed the transfection efficiency of miR-199a-5p agomir. miR-199a-5p overexpression decreased the protein levels of RORγt, a protein that was specifically expressed by Th17 cells [22], pSTAT3, and STAT3 (Fig. 2B, C). Besides, the IL-17 secretion (Fig. 2D) and the proportion of Th17 in CD4+T cells (Fig. 2E, F) were distinctly decreased in response to the miR-199a-5p agomir transfection. These findings suggested that miR-199a-5p overexpression restrained Th17 cell differentiation.
CD4+T cells isolated from spleen tissues of healthy mice were transfected with miR-199a-5p agomir or its negative control (agomir NC) for 48 h, followed by the induction of Th17 differentiation. A miR-199a-5p expression. The protein levels of STAT3, pSTAT3, and retinoic acid receptor-related orphan receptor-gamma-t (RORγt) were detected by western blot. The quantification of band densitometry was shown in (B) and the representative bands were shown in (C). GAPDH and β-actin were used as internal controls. D The IL-17 level was measured in the supernate of CD4+T cells using ELISA. The ratio of Th17 /CD4+T cells was measured using flow cytometry. The quantitative result was shown in ((E); **P < 0.01) and representative histograms were shown in (F). ***P < 0.001 vs. agomir NC.
miR-199a-5p overexpression inhibited Th17 cell differentiation by targeting STAT3
The bioinformatics database forecasted the potential combination of miR-199a-5p and the 3′UTR region of STAT3 mRNA (Fig. 3A). The relative luciferase activity of STAT3 WT, rather than MUT, was notably lessened by miR-199a-5p agomir (Fig. 3A). Next, the miR-199a-5p expression level in CD4+T cells was reinforced by miR-199a-5p agomir transfection and silenced by miR-199a-5p antagomir transfection (Fig. 3B). The STAT3 protein level was decreased by miR-199a-5p overexpression but increased by miR-199a-5p silencing (Fig. 3C). The above data showed that miR-199a-5p targeted STAT3 and decreased its expression in CD4+T cells by binding to its mRNA.
A The above: the potential binding sites between miR-199a-5p and the 3′UTR region of STAT3 mRNA. The below: relative luciferase reporter activities of STAT3 3′UTR wild type (WT) and mutant type (MUT) were detected by dual-luciferase reporter gene assay. B miR-199a-5p expression and C STAT3 expression were detected in CD4+T cells transfected with miR-199a-5p agomir/antagomir/their negative controls (agomir NC or antagomir NC). CD4+T cells were transfected with pMIG-STAT3 or its negative control (pMIG) or agomir NC + pMIG-STAT3 or miR-199a-5p agomir +pMIG-STAT3, 48 h later, CD4+T cells were underwent induction of Th17 differentiation. The protein levels of STAT3, pSTAT3, and RORγt in CD4+T cells were detected by western blot. The quantification of band densitometry was shown in (D) and the representative bands were shown in (E). GAPDH and β-actin were used as internal controls. F The IL-17 level was measured in the supernate of cells using ELISA. The population of Th17 cells was measured in CD4+T cells using flow cytometry. The quantitative result was shown in ((G); ***P < 0.001; ##P < 0.01) and representative histograms were shown in (H). ***P < 0.001 vs. agomir NC or pMIG, #P < 0.05, ##P < 0.01, ###P < 0.001 vs. antagomir NC or agomir NC + STAT3.
The following experiments were conducted to determine whether miR-199a-5p suppressed Th17 differentiation through STAT3. CD4+T cells were transfected with pMIG-STAT3 or pMIG-STAT3 + miR-199a-5p agomir/agomir NC for 48 h, subsequently undergoing the induction of Th17 differentiation. As shown in Fig. 3D, E, pMIG-STAT3 apparently boosted the protein levels of STAT3, pSTAT3, and RORγt, while miR-199a-5p agomir abolished the pMIG-STAT3’s promoting effect on STAT3, pSTAT3, and RORγt expression levels. Additionally, pMIG-STAT3 enhanced IL-17 secretion and augmented the ratio of Th17/CD4+T cells, however, the promoting effect of pMIG-STAT3 on Th17 differentiation was reversed by miR-199a-5p agomir (Fig. 3F–H).
miR-199a-5p-modified ADSCs packaged miR-199a-5p into secreted EVs
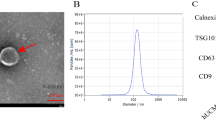
The aforesaid data confirmed the inhibitory influence of miR-199a-5p on Th17 differentiation. However, for it to have this effect, miR-199a-5p must be transferred via a safe and effective vehicle. In this portion of the experiment, we try to use EVs secreted by ADSCs as biological delivery vehicles for miR-199a-5p. First, ADSCs were isolated from healthy mice, and flow cytometry analysis was performed to identify their purity. As shown in Fig. 4A, the isolated ADSCs positively expressed CD90 and CD105 (mesenchymal stromal cell markers [23]) but negatively expressed CD31 and CD45. Meanwhile, the results of Oil Red O staining (Fig. 4B) and Alizarin Red S staining (Fig. 4C) verified that the isolated ADSCs were able to undergo adipogenesis and osteogenesis differentiation when cultured in the corresponding-differentiation mediums. Second, miR-199a-5p agomir or agomir NC was transfected into the ADSCs. Forty-eight hours after transfection, EVs were isolated from the ADSCs supernatant. The morphology and size of EVs isolated from ADSCs transfected with agomir NC (NC-EVs) and EVs isolated from ADSCs transfected with miR-199a-5p agomir (miR-199a-5p-EVs) were shown in Fig. 4D. The results in Fig. 4E showed that both NC-EVs and miR-199a-5p-EVs positively expressed EVs’ markers (CD63 and CD81 [24]). Afterward, the miR-199a-5p expression verified that miR-199a-5p-modified ADSCs efficiently transferred miR-199a-5p into secreted EVs (Fig. 4F).
A The expression levels of surface markers in ADSCs isolated from mice were analyzed using flow cytometry. B After culturing in adipogenesis differentiation medium for 14 days, the adipogenesis differentiation of ADSCs was evaluated by Oil Red staining. Scale bar = 100 μm. C After culturing in osteogenesis differentiation medium for 21 days, the osteogenesis differentiation of ADSCs was evaluated by Alizarin Red S staining. Scale bar = 200 μm. D EVs isolated from ADSCs transfected with agomir NC (NC-EVs) and isolated from ADSCs transfected with miR-199a-5p agomir (miR-199a-5p-EVs) were visualized using Transmission Electron Microscope (TEM). Scale bar = 100 nm. E The expression levels of CD63 and CD81 in miR-199a-5p-EVs and NC-EVs were measured by western blot. Calnexin was used as a negative control. F miR-199a-5p expression was measured by qRT-PCR in miR-199a-5p-EVs and NC-EVs. ***P < 0.001 vs. NC-EVs.
miR-199a-5p-EVs restrained Th17 cell differentiation in vitro
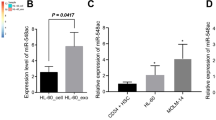
In order to validate whether miR-199a-5p-EVs affected CD4+T cells polarized toward a Th17 phenotype, CD4+T cells were exposed in the Th17 differentiation induction medium combined with miR-199a-5p-EVs or NC-EVs. Relative to cells treated with NC-EVs, cells treated with miR-199a-5p-EVs exhibited an elevated miR-199a-5p level (Fig. 5A) and decreased STAT3, pSTAT3, and RORγt expression levels (Fig. 5B, C). Furthermore, the miR-199a-5p-EVs treatment reduced IL-17 secretion (Fig. 5D), and the ratio of Th17/CD4+T cell (Fig. 5E, F), indicating that EVs isolated from miR-199a-5p-modified ADSCs suppressed Th17 cell differentiation.
CD4+T cells were cultured in Th17 differentiation induction medium combined with miR-199a-5p-EVs or NC-EVs and then harvested for the following tests. A miR-199a-5p expression. The protein levels of STAT3, pSTAT3, and RORγt in CD4+T cells were detected by western blot. The quantification of band densitometry was shown in (B) and the representative bands were shown in (C). GAPDH and β-actin were used as internal controls. D The IL-17 level was measured in the supernate of cells using ELISA. The percentage of Th17 cells was measured in CD4+T cells using flow cytometry. The quantitative result was shown in ((E); ***P < 0.001) and representative histograms were shown in (F). **P < 0.01, ***P < 0.001 vs. CD4+T-NC-EVs.
miR-199a-5p-EVs reduced Th17 cell differentiation in mice with ITP
Finally, in order to examine the impact of miR-199a-5p-EVs on Th17 differentiation in vivo, miR-199a-5p-EVs or NC-EVs were administered to mice with ITP via tail vein injections. As depicted in Fig. 6A, B, in the spleen tissues of miR-199a-5p-EVs-treated mice in the ITP group, the miR-199a-5p level was increased, and the protein levels of STAT3 and pSTAT3 were lessened. Compared with NC-EVs-treated mice in the ITP group, those treated with miR-199a-5p-EVs presented a higher PLT count (Fig. 6C) and a lower serum IL-17 level (Fig. 6D). Furthermore, the reduced proportion of Th17/CD4+T cells in ITP group mice treated with miR-199a-5p-EVs (Fig. 6E, F) confirmed that miR-199a-5p-EVs reduced Th17 cell differentiation in mice with ITP.
BALB/c mice were divided into the ITP-NC-EVs group (n = 8) and the ITP-miR-199a-5p-EVs group (n = 8). A The expression level of miR-199a-5p was detected in the spleen tissues of mice using qRT-PCR. B The expression levels of pSTAT3 and STAT3 were detected in the spleen tissues of mice using western blot. C PLT counts were performed using an automatic blood cell counter on Days 1–8. *P < 0.05. D The serum level of IL-17 was detected using ELISA. The population of Th17 cells was measured in splenic CD4+T cells from mice using flow cytometry. The quantitative result was shown in ((E); **P < 0.01) and representative histograms were shown in (F). **P < 0.01, ***P < 0.001 vs. ITP-NC-EVs.
Discussion
Increasing evidence has shown that the population of Th17 cells is elevated in ITP patients [6], suggesting that the abnormal differentiation of Th17 cells contribute to the occurrence of ITP. Herein this study, we clarified a process by which EVs containing miR-199a-5p can repress Th17 differentiation by targeting STAT3, thus efficiently retarding ITP progression in an experimental ITP mouse model.
The antitumor influence of miR-199a-5p has been verified in several types of cancer [25,26,27]. The latest research has determined that there is a low level of miR-199a-5p expression in ITP patients [5], hinting that miR-199a-5p may take part in ITP pathological process. Running parallel with previous research, in this study, a parent decrease of miR-199a-5p expression was observed in mice with ITP. Subsequently, the in vitro study showed that an elevating miR-199a-5p level could reduce IL-17 level and descend the Th17/CD4+T cell ratio, confirming the negative regulatory influence miR-199a-5p has on Th17 differentiation.
Numerous studies provide evidence that miRNAs repress mRNA expression by binding to the 3′UTR region of target mRNAs [28]. By performing a dual-luciferase reporter gene assay, we determined that miR-199a-5p attenuates STAT3 expression by directly targeting its mRNA. STAT3 is critical for transcriptional regulation of Th17 cell differentiation [11]. On the one hand, under IL-23 stimulation, STAT3 could directly bind to the IL-17A promoter and stimulate its transcription, thus promoting Th17 cell differentiation [29]. On the other hand, STAT3 cooperates with other factors activated by IL-23 or IL-6 in establishing the Th17 phenotype [29]. Multiple studies have proven that inhibiting STAT3 activity or expression can effectively repress Th17 differentiation [30, 31]. In our study, the promoting impact of STAT3 on Th17 differentiation was reversed by miR-199a-5p agomir, confirming that miR-199a-5p suppressed Th17 differentiation by downregulating STAT3 expression. In addition, a decreased pSTAT3 protein level was observed after miR-199a-5p agomir transfection, which suggested that miR-199a-5p also repressed pSTAT3 expression through suppressing STAT3 expression. The target genes of miR-199a-5p predicted by TargetScan were numerous, considering the vital role of STAT3 in Th17 differentiation, we first verified whether miR-199a-5p regulates Th17 differentiation by targeting STAT3. In future work, we will further explore whether other target genes also take part in the regulatory effect of miR-199a-5p on Th17 differentiation.
EVs are nanovesicles released by several cell types and exert their functions as mediators of intercellular communication by transferring protein and RNA. Emerging evidence has shown the therapeutic roles of EVs released from miRNA-modified ADSCs in several diseases, including ischemic stroke, acute myocardial infarction, etc. [32, 33]. However, few reports have addressed EVs’ immunoregulatory role in autoimmune diseases. In the current study, we evaluated the effect of miR-199a-5p-EVs on ITP for the first time. Interestingly, miR-199a-5p-EVs treatment declined IL-17 secretion and the ratio of Th17/CD4+T cells in vitro. What is more, deliveries of miR-199a-5p-EVs into mice in the ITP group markedly increased PLT counts. These beneficial effects produced by miR-199a-5p-EVs were consistent with the impact of miR-199a-5p agomir, confirming that EVs derived from miR-199a-5p-modified ADSCs suppress Th17 differentiation by transferring miR-199a-5p.
In sum, the present data, for the first time, confirms that EVs containing miR-199a-5p repress Th17 differentiation and reveals the potential therapeutic effect of EVs derived from miR-199a-5p-modified ADSCs on ITP.
Change history
18 May 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41374-021-00605-6
References
Cines DB, Blanchette VS. Immune thrombocytopenic purpura. N Engl J Med. 2002;346:995–1008.
Kistangari G, McCrae KR. Immune thrombocytopenia. Hematol Oncol Clin N Am. 2013;27:495–520.
Panitsas FP, Theodoropoulou M, Kouraklis A, Karakantza M, Theodorou GL, Zoumbos NC, et al. Adult chronic idiopathic thrombocytopenic purpura (ITP) is the manifestation of a type-1 polarized immune response. Blood. 2004;103:2645–7.
Noack M, Miossec P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun Rev. 2014;13:668–77.
Zhu X, Ma D, Zhang J, Peng J, Qu X, Ji C, et al. Elevated interleukin-21 correlated to Th17 and Th1 cells in patients with immune thrombocytopenia. J Clin Immunol. 2010;30:253–9.
Qiao J, Li X, Wu Y, Wu X, Zhu F, Liu N, et al. An increased expression profile of Th9/IL-9 correlated with Th17/IL-17 in patients with immune thrombocytopenia. Platelets. 2017;28:287–94.
Li J, Tian J, Lu J, Wang Z, Ling J, Wu X, et al. LncRNA GAS5 inhibits Th17 differentiation and alleviates immune thrombocytopenia via promoting the ubiquitination of STAT3. Int Immunopharmacol. 2020;80:106127.
Jernås M, Nookaew I, Wadenvik H, Olsson B. MicroRNA regulate immunological pathways in T-cells in immune thrombocytopenia (ITP). Blood. 2013;121:2095–8.
Garabet L, Ghanima W, Rangberg A, Teruel-Montoya R, Martinez C, Lozano ML, et al. Circulating microRNAs in patients with immune thrombocytopenia before and after treatment with thrombopoietin-receptor agonists. Platelets. 2020;31:198–205.
Afonso-Grunz F, Müller S. Principles of miRNA-mRNA interactions: beyond sequence complementarity. Cell Mol Life Sci. 2015;72:3127–41.
Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517.
Tyndall A, Uccelli A. Multipotent mesenchymal stromal cells for autoimmune diseases: teaching new dogs old tricks. Bone Marrow Transplant. 2009;43:821–8.
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–28.
Bassi ÊJ, Moraes-Vieira PMM, Moreira-Sá CSR, Almeida DC, Vieira LM, Cunha CS, et al. Immune regulatory properties of allogeneic adipose-derived mesenchymal stem cells in the treatment of experimental autoimmune diabetes. Diabetes. 2012;61:2534–45.
Gonzalez-Rey E, Anderson P, González MA, Rico L, Büscher D, Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58:929–39.
Liang X, Ding Y, Zhang Y, Tse H-F, Lian Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant. 2014;23:1045–59.
Blazquez R, Sanchez-Margallo FM, de la Rosa O, Dalemans W, Alvarez V, Tarazona R, et al. Immunomodulatory potential of human adipose mesenchymal stem cells derived exosomes on in vitro stimulated T cells. Front Immunol. 2014;5:556.
Qu Y, Zhang Q, Cai X, Li F, Ma Z, Xu M, et al. Exosomes derived from miR-181-5p-modified adipose-derived mesenchymal stem cells prevent liver fibrosis via autophagy activation. J Cell Mol Med. 2017;21:2491–502.
Di T-T, Ruan Z-T, Zhao J-X, Wang Y, Liu X, Wang Y, et al. Astilbin inhibits Th17 cell differentiation and ameliorates imiquimod-induced psoriasis-like skin lesions in BALB/c mice via Jak3/Stat3 signaling pathway. Int Immunopharmacol. 2016;32:32–8.
Zhang X, Wang F, Wang Z, Yang X, Yu H, Si S, et al. ALKBH5 promotes the proliferation of renal cell carcinoma by regulating AURKB expression in an m(6)A-dependent manner. Ann Transl Med. 2020;8:646.
Takeda K, Sowa Y, Nishino K, Itoh K, Fushiki S. Adipose-derived stem cells promote proliferation, migration, and tube formation of lymphatic endothelial cells in vitro by secreting lymphangiogenic factors. Ann Plast Surg. 2015;74:728–36.
Yang BH, Hagemann S, Mamareli P, Lauer U, Hoffmann U, Beckstette M, et al. Foxp3(+) T cells expressing RORγt represent a stable regulatory T-cell effector lineage with enhanced suppressive capacity during intestinal inflammation. Mucosal Immunol. 2016;9:444–57.
Castro-Manrreza ME, Bonifaz L, Castro-Escamilla O, Monroy-García A, Cortés-Morales A, Hernández-Estévez E, et al. Mesenchymal stromal cells from the epidermis and dermis of psoriasis patients: morphology, immunophenotype, differentiation patterns, and regulation of T cell proliferation. Stem Cells Int. 2019;2019:4541797.
Vicencio JM, Yellon DM, Sivaraman V, Das D, Boi-Doku C, Arjun S, et al. Plasma exosomes protect the myocardium from ischemia-reperfusion injury. J Am Coll Cardiol. 2015;65:1525–36.
Ma S, Jia W, Ni S. miR-199a-5p inhibits the progression of papillary thyroid carcinoma by targeting SNAI1. Biochem Biophys Res Commun. 2018;497:181–6.
Wang Y, Dai Y-X, Wang S-Q, Qiu M-K, Quan Z-W, Liu Y-B, et al. miR-199a-5p inhibits proliferation and induces apoptosis in hemangioma cells through targeting HIF1A. Int J Immunopathol Pharmacol. 2018;31:394632017749357.
Li Y, Wang D, Li X, Shao Y, He Y, Yu H, et al. MiR-199a-5p suppresses non-small cell lung cancer via targeting MAP3K11. J Cancer. 2019;10:2472–9.
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97.
Mathur AN, Chang H-C, Zisoulis DG, Stritesky GL, Yu Q, O’Malley JT, et al. Stat3 and Stat4 direct development of IL-17-secreting Th cells. J Immunol. 2007;178:4901–7.
Limagne E, Thibaudin M, Euvrard R, Berger H, Chalons P, Végan F, et al. Sirtuin-1 activation controls tumor growth by impeding Th17 differentiation via STAT3 deacetylation. Cell Rep. 2017;19:746–59.
Li Z-H, Wang Y-F, He D-D, Zhang X-M, Zhou Y-L, Yue H, et al. Let-7f-5p suppresses Th17 differentiation via targeting STAT3 in multiple sclerosis. Aging. 2019;11:4463–77.
Geng W, Tang H, Luo S, Lv Y, Liang D, Kang X, et al. Exosomes from miRNA-126-modified ADSCs promotes functional recovery after stroke in rats by improving neurogenesis and suppressing microglia activation. Am J Transl Res. 2019;11:780–92.
Liu J, Jiang M, Deng S, Lu J, Huang H, Zhang Y, et al. miR-93-5p-containing exosomes treatment attenuates acute myocardial infarction-induced myocardial damage. Mol Ther Nucleic Acids. 2018;11:103–15.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 81770115) and the Suzhou Science and Technology Plan Project (Grant No. SZS201808).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The original version of this article unfortunately containd a mistake in an author name. Yanlin Xia should be Yalin Xia.
Rights and permissions
About this article
Cite this article
Li, J., Xia, Y., Fan, X. et al. Extracellular vesicles derived from miR-199a-5p-modified adipose-derived mesenchymal stem cells alleviate immune thrombocytopenia by inhibiting T helper 17 differentiation. Lab Invest 101, 318–327 (2021). https://doi.org/10.1038/s41374-020-00515-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41374-020-00515-z
This article is cited by
-
Therapeutic potential of MSCs and MSC-derived extracellular vesicles in immune thrombocytopenia
Stem Cell Research & Therapy (2023)