Abstract
Transient receptor potential cation channel subfamily V (TRPV) channels play important roles in a variety of cellular processes. One example includes the sensory role of TRPV1 that is sensitive to elevated temperatures and acidic environments and is activated by the hot pepper component capsaicin. Another example is the importance of the highly Ca2+ selective channels TRPV5 and TRPV6 in Ca2+ absorption/reabsorption in the intestine and kidney. However, in some cases such as TRPV4 and TRPV6, breast cancer cells appear to overexpress TRPV channels. Moreover, TRPV mediated Ca2+ influx may contribute to enhanced breast cancer cell proliferation and other processes important in tumor progression such as angiogenesis. It appears that the overexpression of some TRPV channels in breast cancer and/or their involvement in breast cancer cell processes, processes important in the tumor microenvironment or pain may make some TRPV channels potential targets for breast cancer therapy. In this review, we provide an overview of TRPV expression in breast cancer subtypes, the roles of TRPV channels in various aspects of breast cancer progression and consider implications for future therapeutic approaches.
Similar content being viewed by others
Introduction
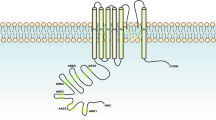
In recent years our appreciation that Ca2+ permeable ion channels have altered expression in some types of cancers, contribute to processes that are critical for cancer progression and may represent targets for cancer therapy has greatly expanded. This is exemplified particularly by the case of transient receptor potential (TRP) cation channels. TRP channels are cation channels, with some members varying greatly in their selectivity for Ca2+ over Na+ ions [1]. TRP channel protein subunits have six transmembrane domains that form tetramers to create the ion channel [1, 2].
Human TRP ion channels can be divided into six subfamilies (TRPA, TRPC, TRPM, TRPML, TRPP, and TRPV). Some have been particularly associated with sensory processes, such as the activation of TRPV1 by heat and the red hot chili component capsaicin, and the sensing of colder temperatures by TRPM8 that is activated by the mint component menthol [3]. The ability of these two ion channels to be activated by exogenous small molecules is indicative of the potential to therapeutically target this family of ion channels. Indeed, TRP channels have been the focus of drug development programs for conditions as diverse as pain, itch, dermatitis, cough, asthma, pulmonary edema [4, 5], and more recently, cancer [6].
TRP channels were one of the first ion channels to be consistently linked to cancer. This association is illustrated by the identification of TRPM8 not first as a cold temperature activated ion channel, but as a protein overexpressed in prostate cancer [7]. In this review we will focus on the transient receptor potential cation channel subfamily V (TRPV) family in breast cancer. We will describe the expression of TRPV channels in breast cancer and review the roles of TRPV channels in breast cancer proliferation, invasiveness and metastasis, cell death, and in the tumor microenvironment (TME). Finally, we will discuss the therapeutic potential of targeting TRPV channels in breast cancer. TRPV channels are an exemplar for ion channels whose different members regulate key aspects of cancer progression and in many instances, these have been shown in models directly relevant to breast cancer [8,9,10,11,12].
The TRPV subfamily
There are six TRPV ion channels (TRPV1-6) with the V standing for vanilloid, a chemical structure class that includes modulators of TRPV channels [13]. Although members of the TRPV family share some characteristics and sequence homology, TRPV5 and TRPV6 are distinguished by their much higher selectivity for Ca2+ over Na+ ions. Below we describe some of the basic properties or distinguishing features of each TRPV channel before describing how these important ion channels intersect with breast cancer.
TRPV1 is a nonselective cation channel and is expressed in a variety of tissues, but its roles in sensory neurons and sensitivity to increases in temperature are particularly well characterized [1]. TRPV1 is activated by a variety of agents including capsaicin (from chili peppers), acidic solutions (H+), vanilloids, and some endocannabiniods [1, 7]. TRPV2 is activated by higher temperatures than TRPV1 (52 vs. 43 °C) [5], it may be located on the endoplasmic reticulum until activation in some cell types, and may have roles in mechanosensing [1, 14]. TRPV3 is a less well understood ion channel, it can also be activated by temperature [15] and a variety of chemical agents including carvacrol, eugenol, and thymol [16] and there has been increasing interest to target it for itch and some skin conditions [17]. An array of TRPV4 activators has been reported including hypotonicity, elevated temperature, and arachidonic acid metabolites [4]. TRPV4 can be sensitized by activation of protease activated receptors and has been reported as having a mechanonsensing role in some cell types [18,19,20]. A variety of drug discovery programs have focused on TRPV4 pharmacological modulators including a clinical evaluation of a TRPV4 inhibitor for pulmonary edema in heart failure [4]. TRPV5 and TRPV6 are arguably a distinct group within the TRPV subfamily because they are more similar to each other than other members of the TRPV family in terms of their amino acid sequence, and their very high selectivity for Ca2+ over Na+ ions, which is not a feature of other TRPV channels [1]. Expression studies and knockout models have shown that TRPV5 and TRPV6 play important roles in Ca2+ fluxes in epithelial cells, and have important roles in Ca2+ absorption from the intestine and Ca2+ reabsorption in the kidney [21].
TRPV channel family expression and breast cancer
The expression levels of a specific calcium channel or pump can be a feature of some cancers. Examples include reduced levels of the Ca2+ pump PMCA4 in colon cancer [22, 23] and elevated TRPM8 in prostate cancer [7]. Moreover, different TRPV channel isoforms have reported altered expression in a variety of cancers including those of the colon, lung, and skin [24,25,26].
The diversity of breast cancer is well established and differences in Ca2+ channel expression can be more evident in specific breast cancer subtypes. The diversity of breast cancer is defined not just by histological grade, but also morphological subtype and the expression of the estrogen (ER), progesterone, and epidermal growth factor (HER2) receptors. Gene expression profiling can also be used to define molecular subtypes of breast cancer such as Basal-like, Claudin-low, HER2-enriched, Luminal A, Luminal B, and Normal-like, which often overlap with the receptor expression profiles and are associated with very different prognosis [27, 28]. Gene expression levels of calcium channels are associated with a breast cancer molecular subtype, such as higher levels of ORAI1 Ca2+ channels in Basal-like breast cancers [29]. Levels of a calcium permeable ion channel can also be associated with prognosis such as the association between TRPM7 and breast cancer metastasis [30]. Below we describe studies that have assessed TRPV subfamily members in breast cancer and breast cancer subtypes.
TRPV1: A study assessing a small number of samples from breast cancers and healthy breast tissue inferred that TRPV1 mRNA levels are elevated in some breast cancers [9]. Our assessment (Fig. 1) suggests that there are no pronounced differences between the breast cancer subtypes. Recently, the localization of TRPV1, in particular the presence of intracellular aggregated TRPV1 protein has been associated with poor patient survival [8]. More studies are required to fully define the association between TRPV1 expression and breast cancer and are warranted given the availability of TRPV1 pharmacological modulators.
Gene expression data were sourced from the METABRIC cohort [55, 56], via cBioPortal for Cancer Genomics [57, 58] and visualized using GraphPad Prism. Gene expression is log2 microarray intensities of 1904 samples (Basal-like 199 samples, Claudin-low 199 samples, HER2-enriched 220 samples, Luminal A 679 samples, Luminal B 461 samples, and Normal-like 140 samples).
TRPV2: Assessment of TRPV2 protein levels using immunohistochemistry (IHC) has shown that there is a much greater proportion of samples with high TRPV2 levels in triple negative breast cancers (ER, PR, and HER2 receptors negative) compared with normal breast tissue and that high levels of TRPV2 are associated with poor prognosis in triple negative breast cancers [10]. Our assessment of TRPV2 in breast molecular subtypes (Fig. 1) also shows higher levels of TRPV2 in the Claudin-low subtype. This is consistent with the aforementioned study given that triple negative breast cancers significantly overlap with this and the Basal-like subtype.
TRPV3: There are no specific reports of TRPV3 in breast cancer, and our analysis (Fig. 1) suggests low levels and no differences between breast cancer molecular subtypes.
TRPV4: Consistent with previous reports [11, 31, 32], Fig. 1 shows that TRPV4 expression is highest in the Basal-like molecular subtype. IHC studies have also shown that TRPV4 expression is higher in breast metastatic lesions than in normal breast tissue and invasive ductal carcinomas, and that TRPV4 expression increases with tumor grade and size [11]. Elevated TRV4 expression is also associated with poorer prognosis and with the Basal-like breast cancer subtype [11].
TRPV5: There are no specific reports of TRPV5 in breast cancer, and our analysis (Fig. 1) suggests low levels and no differences between breast cancer molecular subtypes.
TRPV6: Given the very early report of TRPV6 overexpression in a variety of malignancies [33], it is not surprising that Fig. 1 shows that TRPV6 has the most pronounced and varied overexpression in breast cancers and is highest in the Basal-like and HER2-enriched subtypes. This is consistent with a number of studies reporting elevated TRPV6 in breast cancers including in ER receptor negative breast cancers which have a significant overlap with the Basal-like and HER2-enriched molecular subtypes [12, 34, 35]. In the context of TRPV6, there have been more assessments of the possible mechanisms of TRPV6 overexpression in breast cancer. For example, although perhaps not the main driver of TRPV6 overexpression in breast cancer, TRPV6 copy number is significantly higher in some breast cancer cell lines and in breast cancers identified as ER receptor negative [34].
TRPV channel family and breast cancer cell proliferation
The cancer relevant process that has arguably received the most attention in the context of TRPV channels in breast cancer is proliferation. Ca2+ signaling is an important cell cycle regulator [36] and a variety of calcium channels and pumps have been identified as regulators of proliferation in a variety of cancer cell types using in vitro and in vivo models [37, 38]. The study of effects of TRPV channels on proliferation and also other cancer processes in breast cancer cells using TRPV pharmacological modulators can be unclear when not combined with gene silencing approaches, because some TRPV pharmacological regulators can have off-target effects. Indeed, although studies have reported effects on proliferation of breast cancer cells by TRPV1 pharmacological modulators [39], Chapa et al. have recently reported that some analogs of the TRPV1 antagonist capsazepine could inhibit the proliferation of cancer cell lines in vitro and in vivo despite having no effect on TRPV1 [40], demonstrating that some pharmacological tools may affect cancer cells independently of TRPV channels. Silencing of TRPV6 reduces proliferation in T47D breast cancer cells, a breast cancer cell line which endogenously has high levels of TRPV6 [34, 35]. This antiproliferative effect of TRPV6 silencing in T47D breast cancer cells is associated with reduced accumulation of the S phase marker EdU and apparent altered cell cycle kinetics with increased accumulation in the G1 phase after 24 h serum addition with TRPV6 silencing [34]. The exact mechanism by which TRPV6 silencing reduces breast cancer proliferation is still not fully understood, however, it is interesting to note that TRPV6 silencing reduces levels of AKT phosphorylation in breast cancer cells [41, 42].
TRPV channel family and breast cancer invasiveness and metastasis
There have been far fewer studies on TRPV channels in breast cancer cell migration, invasiveness, and metastasis than proliferation. However, there are some published studies, the most comprehensive of which has focused on TRPV4. TRPV4 silencing in 4T07 cells (a murine mammary cancer cell line that has high level of TRPV4 expression endogenously) reduces wound closure migration, chemotaxis, transendothelial migration, as well as Matrigel© invasion without effecting proliferation [31]. Moreover, exogenous overexpression of TRPV4 promotes cell migration and invasion in a human breast cancer cell line [31]. The relevance of TRPV4 in metastatic processes is further demonstrated by the ability of TRPV4 knockdown to significantly reduce metastatic potential in 4T1 murine breast cancer cells as reflected in the reduced size and number of lung metastasis in vivo [31]. The mechanism by which TRPV4 may contribute to metastasis appears multifactorial with TRPV4 regulating a diverse array of metastasis-relevant processes including breast cancer cell rigidity, AKT signaling, and changes in the cellular adhesion molecule E-cadherin [11, 31]. Given the clinical trials of TRPV4 pharmacological inhibitors in pulmonary edema and heart failure [4], it would now seem appropriate to assess the ability of these agents in reducing metastasis in preclinical breast cancer models. This may offer important drug repurposing opportunities for these TRPV4 inhibitors. There have been some studies focused on other TRPV channels relevant to breast cancer cell migration. These include reports that TRPV2 silencing reduces LL-37 promotion of the migration of MDA-MB-231 and MCF-7 breast cancer cells [43], and reports that TRPV6 silencing does not inhibit the migration of T47D breast cancer cells [34].
TRPV channel family and breast cancer cell death
A number of studies have demonstrated the ability of TRPV silencing and/or pharmacological modulation to promote or induce breast cancer cell death. Both inhibition and activation of TRPV channels could induce or promote cancer cell death, since Ca2+ influx can be a requirement for cellular processes important for cell survival yet excessive calcium levels can be a trigger for cell death [44]. Using tetracycline-inducible overexpression of TRPV1 in MCF-7 breast cancers cells, it was shown that cells with higher levels of TRPV1 are more sensitive to cell death induced by the TRPV1 activator capsaicin [45]. This cell death was correlated with the degree of increase in cytosolic free Ca2+ induced by the TRPV1 activator, was mostly via necrosis and was associated with the upregulation of c-Fos and the necrotic marker RIP3 [45]. Capsaicin has also been reported to induce cell death in SUM149PT breast cancer cells without induced exogenous overexpression of TRPV1 [9] and a positive allosteric modulator of TRPV1 (MRS1477) modestly augments apoptosis markers induced by capsaicin in MCF-7 breast cancer cells [46]. A more pronounced induction of cell death occurs with the pharmacological activation of TRPV4 in breast cancer cell lines that have endogenously high levels of TRPV4 such as MDA-MB-468 breast cancer cells [32]. Treatment of MDA-MB-468 cells with a potent TRPV4 activator GSK1016790A induces oncosis at high concentrations and apoptosis at lower concentrations, which are mediated by TRPV4 as indicated by the attenuation of GSK1016790A-mediated death by TRPV4 silencing [32]. Indeed, breast cancer cell lines that have lower levels (MDA-MB-231) or lack expression of TRPV4 (BT-20) are insensitive to GSK1016790A-induced cell death [32]. More recently, TRPV2 overexpression and pharmacological activation with cannabidiol have been shown to modestly promote doxorubicin-mediated cytotoxic effects in SUM159 and MDA-MB-231 breast cancer cells [10]. The capacity for inhibition of TRPV channels to promote breast cancer cell death has also been shown. Specifically, TRPV6 silencing increases the apoptotic population in T47D breast cancer cells [35] suggesting that the viability of some breast cancer cells with high levels of TRPV6 may be sensitive when constitutively active TRPV6 and hence basal Ca2+ are disrupted.
TRPV channel family and the breast cancer tumor microenvironment
One facet of breast cancer that requires increased attention for all ion channels, not just TRPV channels is the TME [38]. The TME consists of a mix of cells, where TRPV channels may be expressed and play important roles, such as cancer associated fibroblasts, immune cells, and cells of the tumor vasculature and also contains a cocktail of growth factors, reduced oxygen levels, and low pH. These compounding factors could directly or indirectly alter the activity of TRPV channels, as illustrated by the sensitivity of TRPV1 in acidic environments [1]. Likewise, there is potential crosstalk between cellular signals in the TME and TRPV channels, as reported in the vasculature between protease activate receptors, epidermal growth factor receptor, and TRPV4 [47]. However, these and other potential associations have received little attention. For instance, one aspect of the TME which has not yet been explored in the context of TRPV channels is the altered extracellular matrix mechanical properties found in tumors [48].
In the context of breast cancer, there is clear evidence for the importance of TRPV4 in the TME because of its critical role in angiogenesis. Endothelial cells derived from breast cancers have a higher level of TRPV4 than normal controls and are more responsive to TRPV4 activators as assessed by Ca2+ influx assays [49]. More significantly, TRPV4 knockdown greatly reduces arachidonic acid-induced migration in endothelial cells derived from breast cancers, indicating that TRPV4 may be critical in the establishment of the tumor vasculature [49].
Another area where there has been some attention is the association between TRPV channels and cancer pain. For example, there is some evidence that factors that may be present in the bone microenvironment (low pH and formaldehyde) during the metastasis of breast cancer cells to the bone may contribute to bone pain via activation of TRPV1 [50]. Similarly, when rats are injected with a rat mammary gland carcinoma cell line into the bone, there is an upregulation in TRPV1 in the dorsal root ganglion cells which is associated with increased thermal hyperalgesia [51]. These data, combined with reduced movement evoked nociceptive behaviors with TRPV1 pharmacological inhibition and in TRPV1 null mice in an osteolytic sarcoma bone cancer pain model, suggest that TRPV1 may be important in pain associated with breast cancer and breast cancer metastasis [52]. Indeed, MDA-MB-231 breast cancer cells have been shown to promote neuritogenesis and increase neuronal sensitivity to TRPV1 activation [53].
Therapeutic targeting the TRPV channel family in breast cancer
As evident throughout this review, there are potential opportunities in the targeting of TRPV channels to treat different aspects of breast cancer. Pharmacological modulators of specific TRPV channels could potentially have use in reducing breast cancer proliferation, migration, angiogenesis, pain, or could be utilized to promote or induce breast cancer cell death. This diversity is shown in Fig. 2, where the potential to target TRPV4 is illustrated including pharmacological activation of TRPV4 to induce breast cancer cell death in breast cancers that overexpress this TRPV isoform. The potential of agents that target TRPV channels is also exemplified by the clinical trial of a TRPV6 inhibitor for the treatment of a variety of solid tumors including breast cancer [6] and the use of a TRPV6 binding peptide to visualize TRPV6 overexpressing tumors in a preclinical model [54]. The studies on TRPV4 and TRPV6 also highlighted that the overexpression of specific TRPV channels in some breast cancers may allow drugs acting on a TRPV channel isoform to have greater effects in cancer cells compared with normal tissue.
TRPV4 channel is activated by nociception, warmth, hypotonicity, and exogenous small molecules including GSK1016790A, arachidonic acid (AA), and 4α-phorbol 12,13-didecanoate (4αPDD). Angiogenesis: Breast tumor-derived endothelial cells (BTEC) have higher TRPV4 expression compared with human microvascular endothelial cells (HMVEC). Stimulating BTEC with AA or 4αPDD induces TRPV4-actin colocalization, cytoskeletal remodeling, and a migratory phenotype. Metastasis: Expression levels of TRPV4 loosely correlate with the metastatic and migratory potential in murine isogenic mammary cancer cell lines. Stimulating 4T07 cells with 4αPDD increases phospho-AKT and phospho-S6, decreasing E-cadherin and β-catenin. It also increases phospho-FAK and changes the cytoskeletal resulting in an overall decrease in cell softness (*in vivo study was conducted with 4T1 cells). Cell death: A panel of breast cancer cell lines display differential TRPV4 expression levels with the highest levels in MDA-MB-468 cells. Stimulating MDA-MB-468 cells with GSK1016790A induces oncosis and apoptosis. GSK1016790A treatment also decreases intracellular ATP levels, X-linked inhibitor of apoptosis protein (XIAP), and myeloid cell leukemia sequence 1 (Mcl-1) protein. Based on references [11, 31, 32, 49].
Conclusion
TRPV channels differentially contribute to a diverse array of processes in breast cancer cells that are important in breast cancer progression. The clear ability to design pharmacological inhibitors or activators against specific TRPV channels and the marked overexpression of TRPV4 and TRPV6 in some breast cancers illustrate the potential to therapeutically exploit these roles of TRPV channels in breast cancer. Indeed, a variety of studies using pharmacological modulators or gene silencing have now shown the potential of targeting TRPV channels to treat various aspects of this debilitating disease.
References
Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol. 2006;68:619–47.
Clapham DE, Runnels LW, Strubing C. The TRP ion channel family. Nat Rev Neurosci. 2001;2:387–96.
Clapham DE, Montell C, Schultz G, Julius D, International Union of P. International Union of Pharmacology. XLIII. Compendium of voltage-gated ion channels: transient receptor potential channels. Pharmacol Rev. 2003;55:591–6.
Moran MM. TRP channels as potential drug targets. Annu Rev Pharmacol Toxicol. 2018;58:309–30.
Weyer-Menkhoff I, Lotsch J. Human pharmacological approaches to TRP-ion-channel-based analgesic drug development. Drug Discov Today. 2018;23:2003–12.
Fu S, Hirte H, Welch S, Ilenchuk TT, Lutes T, Rice C, et al. First-in-human phase I study of SOR-C13, a TRPV6 calcium channel inhibitor, in patients with advanced solid tumors. Investig New Drugs. 2017;35:324–33.
Tsavaler L, Shapero MH, Morkowski S. Laus R. Trp-p8, a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Cancer Res. 2001;61:3760–9.
Lozano C, Cordova C, Marchant I, Zuniga R, Ochova P, Ramirez-Barrantes R. et al. Intracellular aggregated TRPV1 is associated with lower survival in breast cancer patients. Breast Cancer. 2018;10:161–8.
Weber LV, Al-Refae K, Wolk G, Bonatz G, Altmuller J, Becker C. et al. Expression and functionality of TRPV1 in breast cancer cells. Breast Cancer. 2016;8:243–52.
Elbaz M, Ahirwar D, Xiaoli Z, Zhou X, Lustberg M, Nasser MW, et al. TRPV2 is a novel biomarker and therapeutic target in triple negative breast cancer. Oncotarget. 2018;9:33459–70.
Lee WH, Choong LY, Jin TH, Mon NN, Chong S, Liew CS, et al. TRPV4 plays a role in breast cancer cell migration via Ca(2+)-dependent activation of AKT and downregulation of E-cadherin cell cortex protein. Oncogenesis. 2017;6:e338.
Dhennin-Duthille I, Gautier M, Faouzi M, Guilbert A, Brevet M, Vaudry D, et al. High expression of transient receptor potential channels in human breast cancer epithelial cells and tissues: correlation with pathological parameters. Cell Physiol Biochem. 2011;28:813–22.
Messeguer A, Planells-Cases R, Ferrer-Montiel A. Physiology and pharmacology of the vanilloid receptor. Curr Neuropharmacol. 2006;4:1–15.
Kojima I, Nagasawa M. Trpv2. Handb Exp Pharmacol. 2014;222:247–72.
Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, et al. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature. 2002;418:186–90.
Yang P, Zhu MX. Trpv3. Handb Exp Pharmacol. 2014;222:273–91.
Wang G, Wang K. The Ca(2+)-permeable cation transient receptor potential TRPV3 channel: an emerging pivotal target for itch and skin diseases. Mol Pharmacol. 2017;92:193–200.
Gombedza F, Kondeti V, Al-Azzam N, Koppes S, Duah E, Patil P, et al. Mechanosensitive transient receptor potential vanilloid 4 regulates dermatophagoides farinae-induced airway remodeling via 2 distinct pathways modulating matrix synthesis and degradation. FASEB J. 2017;31:1556–70.
Martinac B, Poole K. Mechanically activated ion channels. Int J Biochem Cell Biol. 2018;97:104–7.
Sostegni S, Diakov A, McIntyre P, Bunnett N, Korbmacher C, Haerteis S. Sensitisation of TRPV4 by PAR2 is independent of intracellular calcium signalling and can be mediated by the biased agonist neutrophil elastase. Pflugers Arch. 2015;467:687–701.
Peng JB, Suzuki Y, Gyimesi G, Hediger MA. TRPV5 and TRPV6 calcium-selective channels. In: Kozak JA, Putney JW Jr., editors. Calcium entry channels in non-excitable cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. p 241–74.
Aung CS, Ye W, Plowman G, Peters AA, Monteith GR, Roberts-Thomson SJ. Plasma membrane calcium ATPase 4 and the remodeling of calcium homeostasis in human colon cancer cells. Carcinogenesis. 2009;30:1962–9.
Ruschoff JH, Brandenburger T, Strehler EE, Filoteo AG, Heinmoller E, Aumuller G, et al. Plasma membrane calcium ATPase expression in human colon multistep carcinogenesis. Cancer Investig. 2012;30:251–7.
Liu X, Zhang P, Xie C, Sham KWY, Ng SSM, Chen Y, et al. Activation of PTEN by inhibition of TRPV4 suppresses colon cancer development. Cell Death Dis. 2019;10:460.
Li X, Zhang Q, Fan K, Li B, Li H, Qi H, et al. Overexpression of TRPV3 correlates with tumor progression in non-small cell lung cancer. Int J Mol Sci. 2016;17:437.
Yang Y, Guo W, Ma J, Xu P, Zhang W, Guo S, et al. Downregulated TRPV1 expression contributes to melanoma growth via the calcineurin-ATF3-p53 pathway. J Investig Dermatol. 2018;138:2205–15.
So CL, Saunus JM, Roberts-Thomson SJ, Monteith GR. Calcium signalling and breast cancer. Semin Cell Dev Biol. 2019;94:74–83.
Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–74.
Azimi I, Milevskiy MJG, Chalmers SB, Yapa K, Robitaille M, Henry C, et al. ORAI1 and ORAI3 in breast cancer molecular subtypes and the identification of ORAI3 as a hypoxia sensitive gene and a regulator of hypoxia responses. Cancers. 2019;11:pii: E208.
Middelbeek J, Kuipers AJ, Henneman L, Visser D, Eidhof I, van Horssen R, et al. TRPM7 is required for breast tumor cell metastasis. Cancer Res. 2012;72:4250–61.
Lee WH, Choong LY, Mon NN, Lu S, Lin Q, Pang B, et al. TRPV4 regulates breast cancer cell extravasation, stiffness and actin cortex. Sci Rep. 2016;6:27903.
Peters AA, Jamaludin SYN, Yapa K, Chalmers S, Wiegmans AP, Lim HF, et al. Oncosis and apoptosis induction by activation of an overexpressed ion channel in breast cancer cells. Oncogene. 2017;36:6490–500.
Zhuang LY, Peng JB, Tou LQ, Takanaga H, Adam RM, Hediger MA, et al. Calcium-selective ion channel, CaT1, is apically localized in gastrointestinal tract epithelia and is aberrantly expressed in human malignancies. Lab Investig. 2002;82:1755–64.
Peters AA, Simpson PT, Bassett JJ, Lee JM, Da Silva L, Reid LE, et al. Calcium channel TRPV6 as a potential therapeutic target in estrogen receptor-negative breast cancer. Mol Cancer Ther. 2012;11:2158–68.
Bolanz KA, Hediger MA, Landowski CP. The role of TRPV6 in breast carcinogenesis. Mol Cancer Ther. 2008;7:271–9.
Roderick HL, Cook SJ. Ca2+ signalling checkpoints in cancer: remodelling Ca2+ for cancer cell proliferation and survival. Nat Rev Cancer. 2008;8:361–75.
Monteith GR, Davis FM, Roberts-Thomson SJ. Calcium channels and pumps in cancer: changes and consequences. J Biol Chem. 2012;287:31666–73.
Monteith GR, Prevarskaya N, Roberts-Thomson SJ. The calcium-cancer signalling nexus. Nat Rev Cancer. 2017;17:367–80.
Vercelli C, Barbero R, Cuniberti B, Racca S, Abbadessa G, Piccione F, et al. Transient receptor potential vanilloid 1 expression and functionality in mcf-7 cells: a preliminary investigation. J Breast Cancer. 2014;17:332–8.
De La Chapa J, Valdez M, Ruiz F 3rd, Gonzales K, Mitchell W, McHardy SF, et al. Synthesis and SAR of novel capsazepine analogs with significant anti-cancer effects in multiple cancer types. Bioorg Med Chem. 2019;27:208–15.
Kaemmerer E, Turner D, Peters AA, Roberts-Thomson SJ, Monteith GR. An automated epifluorescence microscopy imaging assay for the identification of phospho-AKT level modulators in breast cancer cells. J Pharmacol Toxicol Methods. 2018;92:13–9.
Kim SY, Yang D, Myeong J, Ha K, Kim SH, Park EJ, et al. Regulation of calcium influx and signaling pathway in cancer cells via TRPV6-Numb1 interaction. Cell Calcium. 2013;53:102–11.
Gambade A, Zreika S, Gueguinou M, Chourpa I, Fromont G, Bouchet AM, et al. Activation of TRPV2 and BKCa channels by the LL-37 enantiomers stimulates calcium entry and migration of cancer cells. Oncotarget. 2016;7:23785–800.
Monteith GR, McAndrew D, Faddy HM, Roberts-Thomson SJ. Calcium and cancer: targeting Ca2+ transport. Nat Rev Cancer. 2007;7:519–30.
Wu TT, Peters AA, Tan PT, Roberts-Thomson SJ, Monteith GR. Consequences of activating the calcium-permeable ion channel TRPV1 in breast cancer cells with regulated TRPV1 expression. Cell Calcium. 2014;56:59–67.
Naziroglu M, Cig B, Blum W, Vizler C, Buhala A, Marton A, et al. Targeting breast cancer cells by MRS1477, a positive allosteric modulator of TRPV1 channels. PLoS One. 2017;12:e0179950.
Saifeddine M, El-Daly M, Mihara K, Bunnett NW, McIntyre P, Altier C, et al. GPCR-mediated EGF receptor transactivation regulates TRPV4 action in the vasculature. Br J Pharmacol. 2015;172:2493–506.
Acerbi I, Cassereau L, Dean I, Shi Q, Au A, Park C, et al. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr Biol. 2015;7:1120–34.
Fiorio Pla A, Ong HL, Cheng KT, Brossa A, Bussolati B, Lockwich T, et al. TRPV4 mediates tumor-derived endothelial cell migration via arachidonic acid-activated actin remodeling. Oncogene. 2012;31:200–12.
Tong Z, Luo W, Wang Y, Yang F, Han Y, Li H, et al. Tumor tissue-derived formaldehyde and acidic microenvironment synergistically induce bone cancer pain. PLoS One. 2010;5:e10234.
Li Y, Cai J, Han Y, Xiao X, Meng XL, Su L, et al. Enhanced function of TRPV1 via up-regulation by insulin-like growth factor-1 in a rat model of bone cancer pain. Eur J Pain. 2014;18:774–84.
Ghilardi JR, Rohrich H, Lindsay TH, Sevcik MA, Schwei MJ, Kubota K, et al. Selective blockade of the capsaicin receptor TRPV1 attenuates bone cancer pain. J Neurosci. 2005;25:3126–31.
Austin M, Elliott L, Nicolaou N, Grabowska A, Hulse RP. Breast cancer induced nociceptor aberrant growth and collateral sensory axonal branching. Oncotarget. 2017;8:76606–21.
Bowen CV, DeBay D, Ewart HS, Gallant P, Gormley S, Ilenchuk TT, et al. In vivo detection of human TRPV6-rich tumors with anti-cancer peptides derived from soricidin. PLoS One. 2013;8:e58866.
Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486:346–52.
Pereira B, Chin SF, Rueda OM, Vollan HK, Provenzano E, Bardwell HA, et al. The somatic mutation profiles of 2,433 breast cancers refines their genomic and transcriptomic landscapes. Nat Commun. 2016;7:11479.
Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–4.
Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1.
Acknowledgements
This work was supported by Cancer Council Queensland (1159854 and 1139320). GRM was supported by the Mater Foundation. The Translational Research Institute is supported by a grant from the Australian Government. This work makes use of data generated by the Molecular Taxonomy of Breast Cancer International Consortium (METABRIC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
GRM has had projects associated with UniQuest the commercialization company of the University of Queensland.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
So, C.L., Milevskiy, M.J.G. & Monteith, G.R. Transient receptor potential cation channel subfamily V and breast cancer. Lab Invest 100, 199–206 (2020). https://doi.org/10.1038/s41374-019-0348-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41374-019-0348-0
This article is cited by
-
Mechanosensitive TRPV4 channel guides maturation and organization of the bilayered mammary epithelium
Scientific Reports (2024)
-
Pathological mechanisms of cold and mechanical stress in modulating cancer progression
Human Cell (2024)
-
Inhibition of TRPV4 remodels single cell polarity and suppresses the metastasis of hepatocellular carcinoma
Cell Death & Disease (2023)
-
TRP (transient receptor potential) ion channel family: structures, biological functions and therapeutic interventions for diseases
Signal Transduction and Targeted Therapy (2023)
-
RETRACTED ARTICLE: Overexpressed transient receptor potential vanilloid 1 (TRPV1) in lung adenocarcinoma harbours a new opportunity for therapeutic targeting
Cancer Gene Therapy (2022)