Abstract
Liver fibrosis is a worldwide clinical issue. The activation of hepatic stellate cells (HSCs) is the central event during the hepatic fibrotic response. However, the exact mechanisms related to HSC activation and the connection between hepatocytes and HSCs remain unclear. We elucidated the mechanism by which the nuclear-damage-associated molecular pattern molecule, high-mobility group box 1 (HMGB1) was released from the impaired hepatocytes and induced endoplasmic reticulum stress to activate HSCs. In this work, we demonstrated that HMGB1 can be released from hepatocytes and the released HMGB1 activates the HSCs via ER stress at the transcriptional level which was dependent on the activation of both the TLR4 and RAGE signaling pathways rather than the TLR2 signaling pathway. HMGB1 induction of proinflammatory cytokines interleukin (IL)-1β and IL-18 release was dependent on ER stress. In vivo, stable inhibition of HMGB1 suppressed liver fibrosis. These results suggest that under damage condition, HMGB1 can be secreted from injured hepatocytes and activates TLR4- and RAGE signaling pathways to induce ER stress which activates HSCs. Moreover, HMGB1 can produce multiple inflammatory mediators through ER stress, which, in turn, promote liver fibrosis.
Similar content being viewed by others
Introduction
Liver fibrosis is characterized by excessive production and deposition of extracellular matrix, which can be induced by a wide spectrum of chronic liver injuries such as immunological responses, metabolic disorders, infection and toxins [1]. However, until today, there is no effective therapy for liver fibrosis besides the removal of the underlying etiology or liver transplantation. Therefore, a better understanding of liver fibrosis may lead to the identification of new therapeutic targets.
Hepatic stellate cells (HSCs) play a central role in the development of liver fibrosis [2]. Activated HSCs trigger the expression of α-smooth muscle actin (α-SMA) and the production of excessive collagen, along with enhanced proliferation and migration [3], which promote the progression of liver fibrosis. To date, extensive studies have focused on identifying the key factors involved in the pathogenesis of liver fibrosis; however, the precise link between injured hepatocytes and HSCs remains poorly defined.
High-mobility group box1 (HMGB1) is described as a nuclear nonhistone protein involved in DNA replication, repair and energy homeostasis [4]. However, HMGB1 undergoes translocation from the nucleus to the cytoplasm [5]. Extracellular HMGB1 acts as an endogenous danger signal and binds with high affinity to several receptors, including the receptor for Toll-like receptors (TLR)-2/4/9 and receptor for advanced glycation endproducts (RAGE) [6]. Our previous work demonstrated that HMGB1 can promote the metastasis of hepatocellular carcinoma through driving inflammation responses [7]. When released from injured or necrotic cells due to loss of membrane integrity [8] or when secreted by hepatocytes in response to ethanol [9], HMGB1 can trigger harmful responses. Therefore, we hypothesized that extracellular HMGB1 released from the hepatocytes may contribute to live fibrosis through activating HSCs and releasing cytokines.
Numerous genetic and environmental insults interfere with the function of endoplasmic reticulum (ER), leading to the accumulation of unfolded and misfolded proteins in the ER. The unfolded protein response (UPR) is initiated to cope with the resulting ER stress [10]. The UPR pathway activates several proteins, including inositol-requiring enzyme 1 (IRE1) and protein kinase R-like ER kinase (Perk). Moreover, several recent studies revealed that ER stress may mediate the inflammatory response [11,12,13] and liver fibrosis [14, 15]. These data indicate that ER stress may play a critical role in liver fibrosis via several mechanisms.
In this study, we evaluated whether HMGB1 is secreted from hepatocytes and contributes to HSC activation through ER stress. We show that HMGB1 is overexpressed in fibrotic liver and localizes in the cytoplasm. ER stress-related proteins are also highly expressed in liver fibrotic models. HMGB1 can activate HSCs via ER stress. In addition, TLR4 and RAGE, rather than TLR2, play important roles in liver fibrosis and HMGB1-induced ER stress. Moreover, ER stress is involved in the release of interleukin (IL)-1β and IL-18 triggered by HMGB1. The inhibition of HMGB1 suppresses liver fibrosis progression in liver fibrosis models.
Materials and methods
Patient samples, cell lines and reagents
Informed consent was collected at the time of surgical resection at the Tongji Hospital in 2015. The procedure for collection the human samples was approved by the Ethics Committee of the Tongji Hospital, Huazhong University of Science and Technology, and the study was conducted according to the Declaration of Helsinki principles. Cell lines and reagent information are described in the Supporting Materials.
Animal models of liver fibrosis
Thioacetamide (TAA)-induced-fibrosis models
Male Sprague–Dawley rats were purchased from the Department of Experimental Animals of Tongji Medical College. The animals (n = 8) were treated with TAA by intraperitoneal injection two times/week for 4 or 8 weeks to induce chronic liver injury. The control group received an equivalent amount of saline alone (n = 8).
Adenoviral delivery in vivo
Hepatic fibrosis was induced in rats by intraperitoneal injection of TAA two times/week for 6 weeks. Subsequently, each animal received 5 × 109 plaque-forming units (PFUs) of HMGB1 short hairpin RNA (shRNA; AdshHMGB1) or control adenovirus vector (AdshNC) one time/week via the tail vein and it was repeatedly administered TAA for 2 weeks.
The liver tissues and sera were either snap-frozen at −80 °C or fixed in 4% buffered paraformaldehyde for immunostaining. All animal studies were performed in accordance with national and international guidelines. The protocol was approved by the Ethics Committee of Animal Experiments of Tongji Medical College and monitored by the Department of Experimental Animals of the Tongji Medical College.
Immunohistochemistry
Histological analysis of fibrosis was performed on fixed liver tissue that were embedded in paraffin, and sectioned at a thickness of 4 μm. The 4 μm-thick sections were used for hematoxylin and eosin (H&E), Masson’s trichrome and Sirius Red staining.
Immunoblot analysis
Whole-cell proteins were extracted with Cell Lysis Buffer (Sigma-Aldrich). Cytoplasmic protein extraction and western blot analysis were performed following a standard protocol as described previously [7].
ELISA
HMGB1, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels in rat sera were detected by enzyme-linked immunosorbent assay (ELISA; IBL) according to the manufacturer’s instructions.
CCK-8 assay
The cell proliferation was analyzed by Cell Counting Kit-8 (CCK-8) assay according to the manufacturer’s instructions.
Migration assay
Cell migration assays were performed using transwell chambers (8 µm pore size; Corning, China.) Briefly, a total of 5 × 104 cells in 0.4 ml of medium containing recombinant human HMGB1 (rhHMGB1) were plated in the upper chambers, and 600 μl of DMEM medium containing 10% fetal calf serum (Invitrogen Gibco, CA, USA) was added to the lower chamber. After 24 h, the cells that migrated through the membrane to the lower surface were fixed with 4% paraformaldehyde, stained with crystal violet and counted with a microscopy (Olympus, NY, USA).
In vitro wound-repair assay
The cells were seeded in six-well plates overnight to allow for 90% confluency. The cell monolayer was scratched using a sterile 200 μl tip. The growth medium was replaced with serum-free medium supplemented with rhHMGB1. The cell growth in the denuded area was assessed after 24 h. Cell migration was quantified as the percentage of the wound-healed area.
Statistical analysis
The results are expressed as the mean ± SEM. Statistical analysis was performed using Student’s t-test or one-way analysis of variance test. All statistical analyses were performed using Sigma Stat v.3.5 (Systat Software, Inc., Chicago, USA). Graphs were generated using Sigma Plot v.10 (Systat Software, Inc., Chicago, USA). P < 0.05 was denoted as significant.
Results
HMGB1 is overexpressed in fibrotic liver tissues
To study the roles of HMGB1 in fibrotic liver, we first established TAA-induced liver fibrosis animal models confirmed by H&E staining, Masson’s trichrome staining, Sirius red staining (Fig. 1a) and α-SMA, collagen-1 and collagen-3 expression (Fig. 1b). We found that expression of HMGB1 was higher in fibrotic liver compared to the normal control by western blot (Fig. 1c1). We then examined the levels of nuclear and cytoplasmic HMGB1 by the fractionation of nuclear and cytoplasmic proteins in fibrotic liver and normal control liver tissue. The amount of nuclear HMGB1 was not significantly different in fibrotic liver and normal control liver tissues (Fig. 1c2). However, cytoplasmic HMGB1 was absent or was present at low levels in normal control liver tissues, whereas cytoplasmic HMGB1 was found at high levels in fibrotic liver (Fig. 1c3). High cytoplasmic levels of HMGB1 usually occur in the context of active HMGB1 release. Indeed, compared to serum obtained from normal controls, HMGB1 levels were significantly increased in fibrotic models as shown by ELISA analysis (Fig. 1d). To further investigate the localization of HMGB1 in injured livers, we performed immunofluorescence analysis. The results revealed that HMGB1 localized in the cytoplasm of hepatocytes in surgically resected fibrotic liver samples and fibrotic models (Fig. 1e, f). These findings suggest that HMGB1 mainly originate from the hepatocytes and can be actively released into the microenvironment and then stimulate HSCs [16]. Therefore, HMGB1 may play a crucial role in the development of liver fibrosis.
Increased expression of HMGB1 in fibrotic liver. a H&E, Masson’s trichrome staining and Sirius red staining were used to examine the pathological alterations and collagen deposition in TAA-treated rats. b The protein levels of α-SMA, collagen-1 and collagen-3 in fibrotic livers from TAA-treated rats were detected by western blot (n = 2) in each group. c1, c2, c3 The expression of total HMGB1, nuclear HMGB1 and cytoplasmic HMGB1 was measured by western blot (n = 2) in each group. d The expression of HMGB1 in normal and TAA-treated rat sera was detected by ELISA. e The fibrotic liver samples were stained for HMGB1 (green, HMGB1; blue, nuclei). f The normal control (NC) and TAA-treated rats liver tissues were stained for HMGB1 (green, HMGB1; blue, nuclei). **P < 0.01, ***P < 0.0001
HMGB1 induces the activation, proliferation and migration of hepatic stellate cells in vitro
Next, we investigated whether HMGB1 was involved in the process of HSC activation. We first treated LX-2 cells, rat primary HSCs (R-HSC) and HSC-T6 cells with rhHMGB1 (0 ng/ml, 10 ng/ml or 100 ng/ml) for 24 h. Treatment with rhHMGB1 resulted in a significant increase in the expression of α-SMA in a concentration-dependent manner, accompanied by the upregulation of another fibrosis-related gene, collagen-1 (Fig. 2a). Previous research demonstrated that the proliferation and migration rates of activated HSCs were critical to the progression of liver fibrosis [17]. Therefore, we assessed the effect of rhHMGB1 on HSC proliferation and migration. When treated with 10 ng/ml and 100 ng/ml rhHMGB1, LX-2 cell proliferation increased from 0.30 ± 0.02 to 0.56 ± 0.03 and 0.86 ± 0.04, respectively (P < 0.01) (Fig. 2b) as shown by CCK-8 assay. Similarly, the migration capability of LX-2 cells treated with rhHMGB1 was increased compared to the normal control as shown by the transwell assay (Fig. 2c). We then used the wound healing assay to confirm the migration capability (Fig. 2d). In R-HSC (Fig. 2b, c) and HSC-T6 cells (Fig. 2b–d), we obtained similar results. The results illustrate that HMGB1 can induce HSC activation, proliferation and migration.
HMGB1 induces the activation, proliferation and migration of HSCs. a Western blot analysis of α-SMA and collagen-1 in LX-2, R-HSC and HSC-T6 cells treated with various doses of rhHMGB1 for 24 h. b The proliferation rates of LX-2, R-HSC and HSC-T6 cells treated with various dose of rhHMGB1 were determined by CCK-8 assay. c The migration of LX-2, R-HSC and HSC-T6 cells treated with rhHMGB1 was analyzed by transwell assay. d Representative images of the wound-healing assay with LX-2 and HSC-T6 cells treated with various doses of rhHMGB1 for 24 h. *P<0.05, **P<0.01, ***P<0.001.
ER stress is involved in liver fibrosis animal models
To date, some studies have demonstrated that ER stress can induce fibrogenic response in hepatic stellate cells [14]. To study the role of ER stress in vivo, we first used western blot to detect the expression of ER stress-associated proteins (grp78, perk and IRE1α) in fibrotic liver tissue. Grp78, perk and IRE1α were increased in TAA-induced liver fibrosis tissues (Fig. 3a). We performed immunofluorescence double staining to confirm the results in vivo. The results revealed that activated HSCs (α-SMA+ cells) strongly expressed perk, IRE1α and grp78 in TAA-treated rats (Fig. 3b).
ER stress is involved in liver fibrosis. a ER stress-associated proteins Perk, IRE1α and GRP78 were shown by western blot in TAA-induced fibrosis models. b Immunofluorescence co-staining of PERK/IRE1α/grp78 (red) and α-SMA (green) in human cirrhotic liver tissue. c The protein levels of Grp78, α-SMA and collagen-1 in LX-2 and HSC-T6 cells treated with thapsigargin (TG) were determined by western blot. d The protein levels of Grp78, α-SMA and collagen-1 in LX-2 and HSC-T6 cells treated with 4-phenylbutyric acid (4-PBA) were determined by western blot
To further study the roles of ER stress in the activation of HSCs, thapsigargin (TG), the classical ER stress inducer, was used to treat HSCs. TG not only triggered ER stress, but also induced HSC activation, as shown by a strong increase in the protein level of α-SMA and collagen-1 (Fig. 3c), while 4-phenylbutyric acid (4-PBA), an ER stress modulator, led to the downregulation of α-SMA and collagen-1 (Fig. 3d).These findings demonstrate that ER stress may play an important role in liver fibrosis.
HMGB1 can induce ER stress and activate HSCs
The previous results suggest that an association may exist between HMGB1 and ER stress in liver fibrosis. We next sought to study whether HMGB1 participates in the activation of the ER stress. We treated LX-1, R-HSC and HSC-T6 cells with rhHMGB1. RhHMGB1 treatment induced a dose-dependent increase in grp78, perk and IRE1α in these cells as shown by western blot (Fig. 4a). The inhibition of ER stress by 4-PBA can prevent HMGB1-induced activation of HSCs as shown by α-SMA and collagen-1 (Fig. 4b).
HMGB1 can induce ER stress and activate HSCs. a Western blot analysis of PERK, IRE1α and grp78 in LX-2, R-HSC and HSC-T6 cells treated with various doses of rhHMGB1 for 24 h. b LX-2, R-HSC and HSC-T6 cells were treated with rhHMGB1, 4-PBA or rhHMGB1+4-PBA to show the protein expression of α-SMA and collagen-1. c The transwell assay with LX-2, R-HSC and HSC-T6 cells. LX-2, R-HSC and HSC-T6 cells were treated with rhHMGB1 (100 ng/ml), 4-PBA (10 μM) or rhHMGB1 with 4-PBA. Cells were allowed to migrate for 24 h. d The wound-healing assay with LX-2 cells and HSC-T6 cells. LX-2 cells and HSC-T6 cells were stimulated with rhHMGB1 (100 ng/ml), 4-PBA (10 μM) or rhHMGB1 with 4-PBA for 24 h. Cell migration was quantified as a percentage of wound-healed area. e The CCK-8 assay with LX-2, R-HSC and HSC-T6 cells. LX-2, R-HSC and HSC-T6 cells were stimulated with rhHMGB1 (100 ng/ml) or 4-PBA (10 μM) or rhHMGB1 with 4-PBA for 24 h. The proliferation ability was shown by absorbance value (OD). f The expression of PERK, IRE1α and grp78 was detected by real-time PCR. *P < 0.05; **P < 0.01; ***P < 0.0001
We next investigated whether HMGB1-induced ER stress was responsible for the increase in the proliferation and migration triggered by the HMGB1. The proliferation and migration of LX-2 and HSC-T6 cells treated with rhHMGB1 were abolished by the ER stress inhibitor 4-PBA (Fig. 4c–e) as shown by transwell assay, wound healing assay and CCK-8 assay, respectively. These results were confirmed in R-HSC cells (Fig. 4c, e). These results indicate that HMGB1 is required for HSC activation through ER stress.
To characterize the mechanism by which HMGB1 induces ER stress, we treated LX-2 and HSC-T6 cells with rhHMGB1 and measured the IRE1α and perk messenger RNA (mRNA) levels by quantitative real-time reverse transcription-polymerase chain reaction. rhHMGB1 significantly increased the IRE1α and perk mRNA levels in time- and dose-dependent manners (Fig. 4f). These findings suggest that HMGB1 upregulates ER stress-associated proteins at the transcriptional level.
Signaling pathways downstream of HMGB1 are involved in liver fibrosis
Several important receptors have been implicated in HMGB1 signaling, including RAGE, TLR2 and TLR4 [18]. To investigate whether these receptors were involved in liver fibrosis, western blot analysis was performed on whole-cell protein from fibrotic liver tissues. TLR4 and RAGE, but not TLR2, were overexpressed in TAA-induced fibrotic liver tissue (Fig. 5a). To further determine the role of TLR4 in HMGB1-induced activation of HSCs, we used a specific blocking antibody targeted against the TLR4 receptor (a-TLR4). Treatment with a-TLR4 (20 µg/ml) markedly suppressed HMGB1-induced expression of α-SMA and collagen-1 in LX-2 cells (Fig. 5b).
Signaling pathways downstream of HMGB1 are involved in liver fibrosis and ER stress. a TLR4, TLR2 and RAGE receptors were shown by western blot in TAA-induced fibrosis models (n = 2) in each group. b The protein levels of α-SMA and collagen-1 in LX-2 cells treated with anti-TLR4 antibody (a-TLR), goat IgG (IgG), rhHMGB1, rhHMGB1 with a-TLR or rhHMGB1 with IgG were determined by western blot. c, d The protein levels of α-SMA and collagen-1 in LX-2 and HSC-T6 cells treated with anti-RAGE antibody (a-RAGE), goat IgG (IgG), rhHMGB1, rhHMGB1 with a-RAGE or rhHMGB1 with IgG were determined by western blot. e The protein levels of PERK, IRE1α and GRP78 in LX-2 cells treated with anti-TLR4 antibody (a-TLR), goat IgG (IgG), rhHMGB1, rhHMGB1 with a-TLR or rhHMGB1 with IgG were determined by western blot. f, g The protein levels of PERK, IRE1α and GRP78 in LX-2 and HSC-T6 cells treated with anti-RAGE antibody (a-RAGE), goat IgG (IgG), rhHMGB1, rhHMGB1 with a-RAGE or rhHMGB1 with IgG were determined by western blot. h, i LX-2 and HSC-T6 cells were treated with rhHMGB1 (100 ng/ml) or 4-PBA or rhHMGB1+4-PBA to show the protein expression of IL-1β and IL-18
RAGE regulates metabolism, inflammation and epithelial survival in the setting of stress [18]. The RAGE receptor was increased in the fibrotic liver tissues. To further study whether HMGB1-induced activation of HSCs is RAGE dependent, LX-2 and HSC-T6 cells were treated with RAGE receptor antibody (a-RAGE). The expression of α-SMA and collagen-1 were significantly decreased after RAGE inhibition (Fig. 5c, d). These results suggest that HMGB1 activates HSCs through both TLR4 and RAGE signaling pathways.
TLR4 and RAGE receptors are involved in HMGB1-induced ER stress
Our above findings have demonstrated that HMGB1 activates HSCs through TLR4 and RAGE receptors. We next explored whether these receptors are involved in HMGB1-induced ER stress. Treatment with rhHMGB1 with the anti-TLR4 antibody (a-TLR4) in LX-2 cells and with rhHMGB1 and the anti-RAGE antibody (a-RAGE) (20 µg/ml) in LX-2 cell and HSC-T6 cells markedly suppressed HMGB1-induced expression of grp78, perk and IRE1α compared to the normal control (NC) and IgG groups (Fig. 5e–g). These results suggest that HMGB1 induces ER stress through both TLR4 and RAGE signaling pathways and then activates HSCs.
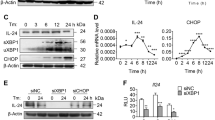
HMGB1 promotes release of IL-1β and IL-18 through ER stress
Chronic inflammation response is the main characteristic of liver fibrosis [19]. We next explored whether HMGB1 can induce inflammation. Treatment with rhHMGB1 (100 ng/ml) can promote the expression of IL-1β and IL-18. Previous studies have demonstrated that ER stress can induce the inflammation response. Therefore, we investigated whether this process was dependent on ER stress. ER stress inhibitor 4-PBA inhibited the release of IL-1β and IL-18 triggered by rhHMGB1 (Fig. 5h, i). The constant inflammation responses may induce production and secretion of other cytokines and chemokines, and then recruit macrophages, T lymphocytes, neutrophils and dendritic cells and then induce liver fibrosis [20].
The inhibition of HMGB1 reduces liver fibrosis in vivo
To determine whether the inhibition of HMGB1 can reduce the fibrogenesis in vivo, we silenced HMGB1 expression in a rat model. Hepatic fibrosis was induced in rats by TAA, and subsequently each animal received AdshHMGB1 or AdshNC via the tail vein for 2 weeks (Fig. 6a). After tail-vein injection of AdshHMGB1, we observed that HMGB1 silencing resulted in reduced liver fibrosis by H&E staining and Masson’s trichrome staining (Fig. 6b, c). Furthermore, α-SMA, collagen-1, perk, IRE1α and grp78 levels were significantly reduced after AdshHMGB1 administration shown by immunohistochemistry and western blot (Fig. 6d–f). In addition, the inhibition of HMGB1 significantly attenuated liver damage as shown by decreased ALT and AST serum levels (Fig. 6g). Taken together, our results showed that HMGB1 silencing interferes with the development of TAA-induced liver fibrosis in vivo.
The inhibition of HMGB1 reduces liver fibrosis in vivo. a Overview of the procedure. Hepatic fibrosis was induced in rats by intraperitoneal injection of TAA two times/week for 6 weeks. Subsequently, each animal received 5 × 109 PFUs of HMGB1 shRNA (AdshHMGB1) or control adenovirus vector (AdshNC) via the tail vein, one time/week and was repetitively administered TAA for 2 weeks. b H&E and Masson’s trichrome staining were used to examine the pathological alterations and collagen deposition in AdshNC group and AdshHMGB1 group. c Semiquantitative analysis of Masson’s trichrome staining in the fibrotic livers from AdshNC- or AdshHMGB1-treated rats made by the Image J (n = 8 rats in each group). d The expression of collagen-1 and α-SMA in the fibrotic livers was analyzed by immunohistochemistry. e Perk, IRE1α and grp78 in the fibrotic livers was analyzed by immunohistochemistry. f The protein levels of PERK, IRE1α, grp78, collagen-1, α-SMA and HMGB1 in the livers were detected by western blot (n = 3) respectively. GAPDH was used as an endogenous control. g Serum biochemical parameters, ALT and AST, were analyzed from AdshNC- or AdshHMGB1-treated rats (n = 8 rats in each group). **P < 0.01, ***P < 0.0001
Discussion
Because liver fibrosis is a mortal disease in the world which is the major cause contributing to the end-stage cirrhosis and hepatocellular carcinoma, there is a pressing need to identify novel targets and explore new therapies to prevent disease onset and/or progression. To date, most of the research in this field has focused on identifying the events involved in the pathogenesis of liver fibrosis; however, the precise link between injured hepatocytes and HSCs remained to be identified. Thus, our goal was to dissect whether HMGB1 can connect hepatocytes and live fibrosis and how the upregulation of HMGB1 contributes to the pathogenesis of liver fibrosis.
This study provides compelling evidence for supporting a direct causal link between HMGB1 release from hepatocytes and liver fibrosis. First, HMGB1 is positively upregulated in rat models with fibrosis and can be secreted from hepatocytes. Second, inhibiting HMGB1 release alleviates liver fibrogenesis in the rat models. Third, rhHMGB1 induces the activation of HSCs by ER stress and this process is dependent on the TLR4 and RAGE rather than the TLR2 signaling pathway and HMGB1 can secrete inflammatory cytokines through ER stress, which may contribute to liver fibrosis.
ER stress has emerged as a common feature of the pathogenesis of diseases associated with fibrosis. For liver fibrosis, ER stress has two opposing effects: apoptosis and adaptation [21, 22]. However, some studies have suggested that ER stress is involved in the progression of liver fibrosis [11, 14]. In this work, we demonstrated that ER stress-associated molecules grp78, perk and IRE1α are involved in TAA-induced fibrosis animal models and these pathways can activate HSCs and induce fibrosis through inflammation response. We show that HMGB1 is the upstream of ER stress and a previous study has also demonstrated that ER stress can induce the release of HMGB1 [23]. These works may show that there is a cycle between ER stress and HMGB1, which can constantly amplify the inflammation response. This may explain the phenomenon in some patients, who had the causative agent removed, resulting in liver cirrhosis.
TLR4 as one of the TLRs is not only important in the regulation of innate and adaptive immune responses [24], but also involved in noninfectious inflammatory diseases [25]. Previous studies have shown that TLR4 is involved in the liver fibrosis [25, 26]. In this work, we reported that TLR4 rather than TLR2 contributes to liver fibrosis as the downstream effector of HMGB1. RAGE is a multiligand receptor that binds structurally diverse molecules, including HMGB1. RAGE activation has been implicated in sterile inflammation as well as in cancer, diabetes and Alzheimer’s disease [18]. We demonstrated that HMGB1 can interact with RAGE and play an important role in the progression of liver fibrosis through inducing the inflammation response. The inhibition of TLR4 and RAGE receptors through neutralizing antibodies can decrease α-SMA and collagen-1 expression and inhibit the ER stress triggered by HMGB1.
In conclusion, the present study indicates that HMGB is positively correlated with HSC activation, proliferation and migration. HMGB1 induces liver fibrosis through ER stress. We also demonstrated that HMGB1 stimulated the HSCs to release cytokines that promote liver fibrosis. The inhibition of HMGB1 can repress liver fibrosis in vivo. Collectively, the evidence suggests that HMGB1may be a therapeutic target in liver fibrosis.
Change history
31 October 2018
Following publication of the original article, the authors noticed some errors in Figs. 1–3. The correct figures can be found in the correction.
References
Kisseleva T, Brenner DA. Mechanisms of fibrogenesis. Exp Biol Med (Maywood). 2008;233:109–22.
Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838–51.
Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425–56.
Kang HJ, Lee H, Choi HJ, et al. Non-histone nuclear factor HMGB1 is phosphorylated and secreted in colon cancers. Lab Invest. 2009;89:948–59.
Taira J, Kida Y, Kuwano K, et al. Protein phosphatase 2A dephosphorylates phosphoserines in nucleocytoplasmic shuttling and secretion of high mobility group box 1. J Biochem. 2013;154:299–308.
Bianchi ME, Manfredi AA. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol Rev. 2007;220:35–46.
Yan W, Chang Y, Liang X, et al. High-mobility group box 1 activates caspase-1 and promotes hepatocellular carcinoma invasiveness and metastases. Hepatology. 2012;55:1863–75.
Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–5.
Ge X, Antoine DJ, Lu Y, Arriazu E, Leung TM, Klepper AL, et al. High mobility group box-1 (HMGB1) participates in the pathogenesis of alcoholic liver disease (ALD). J Biol Chem. 2014;289:22672–91.
Oakes SA, Papa FR. The role of endoplasmic reticulum stress in human pathology. Annu Rev Pathol. 2015;10:173–94.
Li X, Wang Y, Wang H, et al. Endoplasmic reticulum stress is the crossroads of autophagy, inflammation, and apoptosis signaling pathways and participates in liver fibrosis. Inflamm Res. 2015;64:1–7.
Dandekar A, Mendez R, Zhang K. Cross talk between ER stress, oxidative stress, and inflammation in health and disease. Methods Mol Biol. 2015;1292:205–14.
Chaudhari N, Talwar P, Parimisetty A, et al. A molecular web: endoplasmic reticulum stress, inflammation, and oxidative stress. Front Cell Neurosci. 2014;8:213.
Koo JH, Lee HJ, Kim W, et al. Endoplasmic reticulum stress in hepatic stellate cells promotes liver fibrosis via PERK-mediated degradation of HNRNPA1 and up-regulation of SMAD2. Gastroenterology. 2016;150:181–93.
Iracheta-Vellve A, Petrasek J, Gyongyosi B, et al. Endoplasmic reticulum stress-induced hepatocellular death pathways mediate liver injury and fibrosis via stimulator of interferon genes. J Biol Chem. 2016;291:26794–805.
Arriazu E, Ge X, Leung TM, et al. Signalling via the osteopontin and high mobility group box-1 axis drives the fibrogenic response to liver injury. Gut. 2017;66:1123–37.
Brenner DA, Waterboer T, Choi SK, et al. New aspects of hepatic fibrosis. J Hepatol. 2000;32(1 Suppl):32–38.
Sims GP, Rowe DC, Rietdijk ST, et al. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. 2010;28:367–88.
Czaja AJ. Hepatic inflammation and progressive liver fibrosis in chronic liver disease. World J Gastroenterol. 2014;20:2515–32.
Koyama Y, Brenner DA. Liver inflammation and fibrosis. J Clin Invest. 2017;127:55–64.
Gardner BM, Pincus D, Gotthardt K, et al. Endoplasmic reticulum stress sensing in the unfolded protein response. Cold Spring Harb Perspect Biol. 2013;5:a13169.
Malhotra JD, Kaufman RJ. ER stress and its functional link to mitochondria: role in cell survival and death. Cold Spring Harb Perspect Biol. 2011;3:a4424.
Park IA, Heo SH, Song IH, et al. Endoplasmic reticulum stress induces secretion of high-mobility group proteins and is associated with tumor-infiltrating lymphocytes in triple-negative breast cancer. Oncotarget. 2016;7:59957–64.
Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511.
Seki E, De Minicis S, Osterreicher CH, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–32.
Seifert L, Deutsch M, Alothman S, et al. Dectin-1 regulates hepatic fibrosis and hepatocarcinogenesis by suppressing TLR4 signaling pathways. Cell Rep. 2015;13:1909–21.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 81472311, No. 81772607) and Science Foundation for The Excellent Youth of Tongji hospital (No: 2016YQ06).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
He, Q., Fu, Y., Ding, X. et al. High-mobility group box 1 induces endoplasmic reticulum stress and activates hepatic stellate cells. Lab Invest 98, 1200–1210 (2018). https://doi.org/10.1038/s41374-018-0085-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41374-018-0085-9
This article is cited by
-
HMGB1 promotes mitochondrial transfer between hepatocellular carcinoma cells through RHOT1 and RAC1 under hypoxia
Cell Death & Disease (2024)
-
Hepatic HRC induces hepatocyte pyroptosis and HSCs activation via NLRP3/caspase-1 pathway
Journal of Molecular Medicine (2022)
-
Auranofin prevents liver fibrosis by system Xc-mediated inhibition of NLRP3 inflammasome
Communications Biology (2021)
-
Reprogramming of microRNA expression via E2F1 downregulation promotes Salmonella infection both in infected and bystander cells
Nature Communications (2021)
-
ER stress activates immunosuppressive network: implications for aging and Alzheimer’s disease
Journal of Molecular Medicine (2020)