Abstract
Objective
To examine the change in CO2, when applying NIPPV with either a low or a high rate in stable premature infants.
Study design
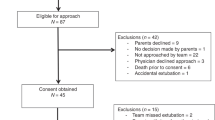
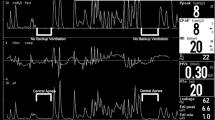
Prospective, controlled, crossover study. Preterm infants on NIPPV were monitored by tcCO2 during two rate changes switching every hour between high (30 bpm) and low (10 bpm) set rates.
Results
Fifty premature infants (mean ± SD: 28.3 ± 2.4 weeks’ gestation) were enrolled. Each infant had two rate changes; therefore, a hundred rate changes were studied. The mean change in tcCO2, i.e., ΔtcCO2 (95% confidence-interval), was −1.1 (−2.3 to 0.1) mmHg for increasing rate from low to high, and 0.46 (−0.49 to 1.41) mmHg for decreasing rate from high to low.
Conclusion
Multiplying or dividing the rate settings by three did not significantly change the tcCO2 readings an hour after the change. These findings could affect the management of ventilation settings of NIPPV in premature infants.
Clinical trial registry
ClinicalTrials.gov ID: NCT04836689, The name of the trial registry: “Influence of Respiratory Rate Settings on CO2 Levels During Nasal Intermittent Positive Pressure Ventilation (NIPPV).”
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Garg S, Sinha S. Non-invasive Ventilation in Premature Infants: Based on Evidence or Habit. J Clin Neonatol. 2013;2:155–9.
Lemyre B, Laughon M, Bose C, Davis PG. Early nasal intermittent positive pressure ventilation (NIPPV) versus early nasal continuous positive airway pressure (NCPAP) for preterm infants. Cochrane Database of Systematic Reviews. 2016. https://doi.org/10.1002/14651858.CD005384.pub2.
Lemyre B, Davis PG, de Paoli AG, Kirpalani H Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for preterm neonates after extubation. Cochrane Database of Systematic Reviews 2017. https://doi.org/10.1002/14651858.CD003212.pub3.
Kugelman A, Feferkorn I, Riskin A, Chistyakov I, Kaufman B, Bader D Nasal Intermittent Mandatory Ventilation Versus Nasal Continuous Positive Airway Pressure for Respiratory Distress Syndrome: A Randomized, Controlled, Prospective Study. J Pediatrics. 2007. https://doi.org/10.1016/j.jpeds.2007.01.032.
Lemyre B, Deguise M-O, Benson P, Kirpalani H, Ekhaguere O, Davis PG Early nasal intermittent positive pressure ventilation versus early nasal continuous positive airway pressure for preterm infants. (Publication Number: 3180.4). In: Pediatric Academic Societies (PAS) Meeting. 2022.
Widdicombe J. Henry Head and his paradoxical reflex. J Physiol. 2004;559:1–2.
Owen LS, Morley CJ, Davis PG. Neonatal nasal intermittent positive pressure ventilation: What do we know in 2007? Arch Dis Child Fetal Neonatal Ed. 2007;92:414–8.
Khalaf MN, Brodsky N, Hurley J, Bhandari V. A prospective randomized, controlled trial comparing synchronized nasal intermittent positive pressure ventilation versus nasal continuous positive airway pressure as modes of extubation. Pediatrics. 2001;108:13–7.
Piper AJ, Sullivan CE. Effects of short-term NIPPV in the treatment of patients with severe obstructive sleep apnea and hypercapnia. Chest. 1994;105:434–40.
Kiciman NM, André B, Bernstein G, Mannino FL, Rich W, Henderson C, et al. Thoracoabdominal Motion in Newborns During Ventilation Delivered by Endotracheal Tube or Nasal Prongs. Pediatr Pulmonol. 1998;25:175–81.
Moretti C, Gizzi C, Papoff P, Lampariello S, Capoferri M, Calcagnini G, et al. Comparing the effects of nasal synchronized intermittent positive pressure ventilation (nSIPPV) and nasal continuous positive airway pressure (nCPAP) after extubation in very low birth weight infants. Early Hum Dev. 1999;56:167–77.
Aghai ZH, Saslow JG, Nakhla T, Milcarek B, Hart J, Lawrysh-Plunkett R, et al. Synchronized nasal intermittent positive pressure ventilation (SNIPPV) decreases work of breathing (WOB) in premature infants with respiratory distress syndrome (RDS) compared to nasal continuous positive airway pressure (NCPAP). Pediatr Pulmonol. 2006;41:875–81.
Kirpalani H, Millar D, Lemyre B, Yoder BA, Chiu A, Roberts RS. A trial comparing noninvasive ventilation strategies in preterm infants. N Engl J Med. 2013. https://doi.org/10.1056/NEJMoa1214533.
Newborn Services Clinical Practice Committee. Nasal Intermittent Positive Pressure Ventilation (NIPPV). 2018.https://www.starship.org.nz/guidelines/nasal-intermittent-positive-pressure-ventilation-nippv/ (accessed 1 Sep2022).
Meneses J, Bhandari V, Alves JG, Herrmann D. Noninvasive ventilation for respiratory distress syndrome: a randomized controlled trial. Pediatrics. 2011;127:300–7.
Shi Y, Tang S, Zhao J, Shen J. A prospective, randomized, controlled study of NIPPV versus nCPAP in preterm and term infants with respiratory distress syndrome. Pediatr Pulmonol. 2014;49:673–8.
Chakkarapani AA, Adappa R, Mohammad Ali SK, Gupta S, Soni NB, Chicoine L, et al. “Current concepts of mechanical ventilation in neonates” – Part 1: Basics. Int J Pediatr Adolesc Med. 2020;7:15–20.
Gizzi C, Montecchia F, Panetta V, Castellano C, Mariani C, Campelli M, et al. Is synchronised NIPPV more effective than NIPPV and NCPAP in treating apnoea of prematurity (AOP)? A randomised cross-over trial. Arch Dis Child Fetal Neonatal Ed. 2015;100:F17–F23.
Borenstein-Levin L, Konikoff L, Solimano A. Clinical quantification of SpO(2) instability using a new histogram classification system: a clinical study. Pediatr Res. 2020;87:716–20.
Hochwald O, Riskin A, Borenstein-Levin L, Shoris I, Dinur GP, Said W, et al. Cannula With Long and Narrow Tubing vs Short Binasal Prongs for Noninvasive Ventilation in Preterm Infants: Noninferiority Randomized Clinical Trial. JAMA Pediatr. 2021;175:36–43.
Julious SA. Sample sizes for clinical trials with normal data. Stat Med. 2004;23:1921–86.
Sealed Envelope Ltd. Power calculator for continuous outcome equivalence trial. [Online] Available from: https://www.sealedenvelope.com/power/continuous-equivalence/ [Accessed Fri Jul 17 2020] 2012.
Piaggio G, Elbourne DR, Altman DG, Pocock SJ, Evans SJW. Reporting of noninferiority and equivalence randomized trials: An extension of the CONSORT statement. J Am Med Assoc. 2006. https://doi.org/10.1001/jama.295.10.1152.
Hochwald O, Borenstein-Levin L, DInur G, Jubran H, Ben-David S, Kugelman A Continuous noninvasive carbon dioxide monitoring in neonates: From theory to standard of care. Pediatrics 2019; 144. https://doi.org/10.1542/peds.2018-3640.
Owen LS, Morley CJ, Dawson JA, Davis PG. Effects of non-synchronised nasal intermittent positive pressure ventilation on spontaneous breathing in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2011;96:F422–8.
Matlock DN, Bai S, Weisner MD, Comtois N, Beck J, Sinderby C et al. Tidal volume transmission during non-synchronized nasal intermittent positive pressure ventilation via RAM® cannula. J Perinatol 2019. https://doi.org/10.1038/s41372-019-0333-x.
Sehgal IS, Dhooria S, Aggarwal AN, Behera D, Agarwal R. Asynchrony index in pressure support ventilation (PSV) versus neurally adjusted ventilator assist (NAVA) during non-invasive ventilation (NIV) for respiratory failure: systematic review and meta-analysis. Intensive Care Med. 2016;42:1813–5.
Stella MH, England SJ. Modulation of laryngeal and respiratory pump muscle activities with upper airway pressure and flow. J Appl Physiol (1985). 2001;91:897–904.
Oppersma E, Doorduin J, van der Heijden EHFM, van der Hoeven JG, Heunks LMA Noninvasive ventilation and the upper airway: should we pay more attention? Crit Care 2013; 17. https://doi.org/10.1186/CC13141.
Cresi F, Chiale F, Maggiora E, Borgione SM, Ferroglio M, Runfola F, et al. Short-term effects of synchronized vs. non-synchronized NIPPV in preterm infants: study protocol for an unmasked randomized crossover trial. Trials. 2021;22:392.
Author information
Authors and Affiliations
Contributions
HO conceptualized and designed the study, designed the data collection instruments, collected data, carried out the initial analyses, drafted the initial manuscript, and revised the manuscript. B-LL and KA conceptualized and designed the study, and critically reviewed and revised the manuscript. DG, JH, LY, and BM collected data, carried out the initial analyses, and critically reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hochwald, O., Borenstein-Levin, L., Dinur, G. et al. The effect of changing respiratory rate settings on CO2 levels during nasal intermittent positive pressure ventilation (NIPPV) in premature infants. J Perinatol 43, 305–310 (2023). https://doi.org/10.1038/s41372-023-01614-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-023-01614-7