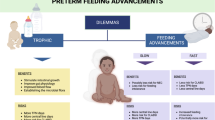
Abstract
With limited clinical evidence available to guide common nutritional decisions, significant variation exists in approaches to enteral feeding for very preterm infants, specifically when feedings are initiated, what is fed, and the method used for feedings. Preclinical studies have highlighted the benefits associated with avoiding nil per os and providing early-stage mother’s own milk or colostrum. However, these recommended approaches are often mutually exclusive due to the delays in lactation associated with very preterm delivery, resulting in uncertainty regarding which approach should be prioritized. Few studies have evaluated feeding frequency in preterm infants, with limited generalizability to extremely preterm infants. Therefore, even evidence-based approaches to very preterm infant feed initiation can differ. Future research is needed to identify optimal strategies for enteral nutrition in very preterm infants, but, until then, evidence-informed approaches may vary depending on each neonatal intensive care unit’s assessment of risk and benefit.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
McLeod G, Farrent S, Gilroy M, Page D, Oliver CJ, Richmond F, et al. Variation in neonatal nutrition practice and implications: a survey of Australia and New Zealand neonatal units. Front Nutr. 2021;8:642474.
Nommsen-Rivers LA, Chantry CJ, Peerson JM, Cohen RJ, Dewey KG. Delayed onset of lactogenesis among first-time mothers is related to maternal obesity and factors associated with ineffective breastfeeding. Am J Clin Nutr. 2010;92:574–84.
Neville MC, Anderson SM, McManaman JL, Badger TM, Bunik M, Contractor N, et al. Lactation and neonatal nutrition: defining and refining the critical questions. J Mammary Gland Biol Neoplasia. 2012;17:167–88.
Hoban R, Bowker RM, Gross ME, Patel AL. Maternal production of milk for infants in the neonatal intensive care unit. Semin Perinatol. 2021;45:151381.
Parker LA, Sullivan S, Kruger C, Mueller M. Timing of milk expression following delivery in mothers delivering preterm very low birth weight infants: a randomized trial. J Perinatol. 2020;40:1236–45.
Wagner CL, Taylor SN, Johnson D. Host factors in amniotic fluid and breast milk that contribute to gut maturation. Clin Rev Allergy Immunol. 2008;34:191–204.
Shulman RJ, Schanler RJ, Lau C, Heitkemper M, Ou C, Smith EO. Early feeding, antenatal glucocorticoids, and human milk decrease intestinal permeability in preterm infants. Pediatr Res. 1998;44:519–23.
Rouwet EV, Heineman E, Buurman WA, ter Riet G, Ramsay G, Blanco CE. Intestinal permeability and carrier-mediated monosaccharide absorption in preterm neonates during the early postnatal period. Pediatr Res. 2002;51:64–70.
Taylor SN, Basile LA, Ebeling M, Wagner CL. Intestinal permeability in preterm infants by feeding type: Mother’s milk versus formula. Breastfeed Med. 2009;4:11–5.
Quigley M, Embleton ND, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev. 2019;7:CD002971.
Young L, Oddie SJ, McGuire W. Delayed introduction of progressive enteral feeds to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database Syst Rev. 2022;1:CD001970.
Viswanathan S, Merheb R, Wen X, Collin M, Groh-Wargo S. Standardized slow enteral feeding protocol reduces necrotizing enterocolitis in micropremies. J Neonatal Perinat Med. 2017;10:171–80.
Hamilton E, Massey C, Ross J, Taylor S. Early enteral feeding in very low birth weight infants. Early Hum Dev. 2014;90:227–30.
Salas AA, Li P, Parks K, Lal CV, Martin CR, Carlo WA. Early progressive feeding in extremely preterm infants: a randomized trial. Am J Clin Nutr. 2018;107:365–70.
Morgan J, Bombell S, McGuire W. Early trophic feeding versus enteral fasting for very preterm or very low birth weight infants. Cochrane Database Syst Rev. 2013:CD000504.
Sangild PT, Thymann T, Schmidt M, Stoll B, Burrin DG, Buddington RK. Invited review: the preterm pig as a model in pediatric gastroenterology. J Anim Sci. 2013;91:4713–29.
Konnikova Y, Zaman MM, Makda M, D’Onofrio D, Freedman SD, Martin CR. Late enteral feedings are associated with intestinal inflammation and adverse neonatal outcomes. PLoS One. 2015;10:e0132924.
Sallakh-Niknezhad A, Bashar-Hashemi F, Satarzadeh N, Ghojazadeh M, Sahnazarli G. Early versus late trophic feeding in very low birth weight preterm infants. Iran J Pediatr. 2012;22:171–6.
Jajoo M, Singh A, Arora N, Bhaskar V, Mandal A. Early total versus gradually advanced enteral nutrition in stable very-low-birth-weight preterm neonates: a randomized, controlled trial. Indian J Pediatr. 2022;89:25–30.
Nangia S, Vadivel V, Thukral A, Saili A. Early total enteral feeding versus conventional enteral feeding in stable very-low-birth-weight infants: a randomised controlled trial. Neonatology. 2019;115:256–62.
Sanghvi KP, Joshi P, Nabi F, Kabra N. Feasibility of exclusive enteral feeds from birth in VLBW infants >1200 g—an RCT. Acta Paediatr. 2013;102:e299–e304.
Hoban R, Medina Poeliniz C, Somerset E, Tat Lai C, Janes J, Patel AL, et al. Mother’s own milk biomarkers predict coming to volume in pump-dependent mothers of preterm infants. J Pediatr. 2021;228:P44–P52.e3.
Rasmussen SO, Martin L, Ostergaard MV, Rudloff S, Li Y, Roggenbuck M, et al. Bovine colostrum improves neonatal growth, digestive function, and gut immunity relative to donor human milk and infant formula in preterm pigs. Am J Physiol Gastrointest Liver Physiol. 2016;311:G480–91.
Tully DB, Jones F, Tully MR. Donor milk: what’s in it and what’s not. J Hum Lact. 2001;17:152–5.
Bertino E, Coppa GV, Giuliani F, Coscia A, Gabrielli O, Sabatino G, et al. Effects of holder pasteurization on human milk oligosaccharides. Int J Immunopathol Pharm. 2008;21:381–5.
Ewaschuk JB, Unger S, Harvey S, O’Connor DL, Field CJ. Effect of pasteurization on immune components of milk: Implications for feeding preterm infants. Appl Physiol Nutr Metab. 2011;36:175–82.
Peila C, Moro GE, Bertino E, Cavallarin L, Giribaldi M, Giuliani F, et al. The effect of holder pasteurization on nutrients and biologically-active components in donor human milk: a review. Nutrients. 2016;8:477.
Ley SH, Hanley AJ, Stone D, O’Connor DL. Effects of pasteurization on adiponectin and insulin concentrations in donor human milk. Pediatr Res. 2011;70:278–81.
Hanson C, Lyden E, Furtado J, Van Ormer M, Anderson-Berry A. A comparison of nutritional antioxidant content in breast milk, donor milk, and infant formulas. Nutrients. 2016;8:E681.
Piemontese P, Mallardi D, Liotto N, Tabasso C, Menis C, Perrone M, et al. Macronutrient content of pooled donor human milk before and after holder pasteurization. BMC Pediatr. 2019;19:58.
Good M, Sodhi CP, Hackam DJ. Evidence based feeding strategies before and after the development of necrotizing enterocolitis. Expert Rev Clin Immunol. 2014;10:875–84.
Good M, Siggers RH, Sodhi CP, Afrazi A, Alkhudari F, Egan CE, et al. Amniotic fluid inhibits toll-like receptor 4 signaling in the fetal and neonatal intestinal epithelium. Proc Natl Acad Sci USA. 2012;109:11330–5.
Lemay DG, Ballard OA, Hughes MA, Morrow AL, Horseman ND, Nommsen-Rivers LA. RNA sequencing of the human milk fat layer transcriptome reveals distinct gene expression profiles at three stages of lactation. PLoS One. 2013;8:e67531.
Sangild PT, Vonderohe C, Melendez Hebib V, Burrin DG. Potential benefits of bovine colostrum in pediatric nutrition and health. Nutrients. 2021;13:2551.
Noel G, In JG, Lemme-Dumit JM, DeVine LR, Cole RN, Guerrerio AL, et al. Human breast milk enhances intestinal mucosal barrier function and innate immunity in a healthy pediatric human enteroid model. Front Cell Dev Biol. 2021;9:685171.
Castellote C, Casillas R, Ramírez-Santana C, Pérez-Cano FJ, Castell M, Moretones MG, et al. Premature delivery influences the immunological composition of colostrum and transitional and mature human milk. J Nutr. 2011;141:1181–7.
Hassiotou F, Beltran A, Chetwynd E, Stuebe AM, Twigger AJ, Metzger P, et al. Breastmilk is a novel source of stem cells with multilineage differentiation potential. Stem Cells. 2012;30:2164–74.
Pannaraj PS, Li F, Cerini C, Bender JM, Yang S, Rollie A, et al. Association between breast milk bacterial communities and establishment and development of the infant gut microbiome. JAMA Pediatr. 2017;171:647–54.
Weström B, Arévalo Sureda E, Pierzynowska K, Pierzynowski SG, Pérez-Cano FJ. The immature gut barrier and its importance in establishing immunity in newborn mammals. Front Immunol. 2020;11:1153.
Jensen AR, Elnif J, Burrin DG, Sangild PT. Development of intestinal immunoglobulin absorption and enzyme activities in neonatal pigs is diet dependent. J Nutr. 2001;131:3259–65.
Cristofalo EA, Schanler RJ, Blanco CL, Sullivan S, Trawoeger R, Kiechl-Kohlendorfer U, et al. Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. J Pediatr. 2013;163:1592–5.
Sadrudin Premji S, Chessell L, Stewart F. Continuous nasogastric milk feeding versus intermittent bolus milk feeding for preterm infants less than 1500 grams. Cochrane Database Syst Rev. 2021;6:CD001819.
Rogers SP, Hicks PD, Hamzo M, Veit LE, Abrams SA. Continuous feedings of fortified human milk lead to nutrient losses of fat, calcium and phosphorous. Nutrients. 2010;2:230–40.
World Health Organization. Guidelines on optimal feeding of low birth-weight infants in low- and middle-income countries. Geneva: World Health Organization; 2011.
Anushree N, Shaw S, Negi V. 2 hourly versus 3 hourly feeding schedule in very low birth weight preterm neonates. J Mar Med Soc. 2018;20:96–9.
Unal S, Demirel N, Bas AY, Arifoğlu İ, Erol S, Ulubas Isik D. Impact of feeding interval on time to achieve full oral feeding in preterm infants: a randomized trial. Nutr Clin Pr. 2019;34:783–8.
Yadav A, Siddiqui N, Debata PK. Two-hourly vs three-hourly feeding in very low birthweight neonates: a randomized controlled trial. Indian Pediatr. 2021;58:320–4.
Dhingra A, Agrawal SK, Kumar P, Narang A. A randomised controlled trial of two feeding schedules in neonates weighing ≤1750 g. J Matern Fetal Neonatal Med. 2009;22:198–203.
Ibrahim NR, Kheng TH, Nasir A, Ramli N, Foo JLK, Syed Alwi SH, et al. Two-hourly versus 3-hourly feeding for very low birthweight infants: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2017;102:F225–9.
Tali S, Kabra N, Ahmad J, Dash SK, Balasubramanian H, Avasthi BS, et al. Effect of feeding schedule on time to reach full feeds in ELBW and VLBW neonates: a randomized trial. Perinatology. 2016;17:95–102.
Kumar J, Meena J, Debata P, Sankar M, Kumar P, Shenoi A Three-hourly versus two-hourly feeding interval in stable preterm infants: an updated systematic review and meta-analysis of randomized controlled trials. Eur J Pediatr. 2022;181:2075–86.
Leaf A, Dorling J, Kempley S, McCormick K, Mannix P, Linsell L, et al. Early or delayed enteral feeding for preterm growth-restricted infants: a randomized trial. Pediatrics. 2012;129:e1260–8.
Jung E, Romero R, Yeo L, Diaz-Primera R, Marin-Concha J, Para R, et al. The fetal inflammatory response syndrome: the origins of a concept, pathophysiology, diagnosis, and obstetrical implications. Semin Fetal Neonatal Med. 2020;25:101146.
Elgin TG, Fricke EM, Gong H, Reese J, Mills DA, Kalantera KM, et al. Fetal exposure to maternal inflammation interrupts murine intestinal development and increases susceptibility to neonatal intestinal injury. Dis Model Mech. 2019;12:dmm040808.
Azad MB, Konya T, Persaud RR, Guttman DS, Chari RS, Field CJ, et al. Impact of maternal intrapartum antibiotics, method of birth and breastfeeding on gut microbiota during the first year of life: a prospective cohort study. BJOG. 2016;123:983–93.
Roberts D, Brown J, Medley N, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2017;3:CD004454.
Yang J, Hou L, Wang J, Xiao L, Zhang J, Yin N, et al. Unfavourable intrauterine environment contributes to abnormal gut microbiome and metabolome in twins. Gut. 2022;71:2451–62.
Olszewski J, Zabielski R, Skrzypek T, Matyba P, Wierzbicka M, Adamski A, et al. Differences in intestinal barrier development between intrauterine growth restricted and normal birth weight piglets. Animals (Basel). 2021;11:990.
Author information
Authors and Affiliations
Contributions
ALP was responsible for the conception, design, drafting, and review of the manuscript, and approved the final version. SNT was responsible for the conception, design, drafting, and review of the manuscript, and approved the final version.
Corresponding author
Ethics declarations
Competing interests
ALP has no competing interests. SNT has received an honorarium from Astarte Medical and a consultation fee from Alcresta Therapeutics.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Patel, A.L., Taylor, S.N. Dilemmas in initiation of very preterm infant enteral feeds—when, what, how?. J Perinatol 43, 108–113 (2023). https://doi.org/10.1038/s41372-022-01564-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-022-01564-6