Abstract
Objective
We aimed to determine whether coronavirus-disease-2019 (COVID-19) pandemic exposure duration was associated with PTB and if the pandemic modified racial disparities.
Study design
We analyzed Philadelphia births and replicated in New Haven. Compared to matched months in two prior years, we analyzed overall PTB, specific PTB phenotypes, and stillbirth.
Results
Overall, PTB was similar between periods with the following exceptions. Compared to pre-pandemic, early pregnancy (<14 weeks’) pandemic exposure was associated with lower risk of PTB < 28 weeks’ (aRR 0.60 [0.30–1.10]) and later exposure with higher risk (aRR 1.77 [0.78–3.97]) (interaction p = 0.04). PTB < 32 weeks’ among White patients decreased during the pandemic, resulting in non-significant widening of the Black-White disparity from aRR 2.51 (95%CI: 1.53–4.16) to aRR 4.07 (95%CI: 1.56–12.01) (interaction P = 0.41). No findings replicated in New Haven.
Conclusion
We detected no overall pandemic effects on PTB, but potential indirect benefits for some patients which could widen disparities remains possible.
Similar content being viewed by others
Introduction
While pregnant people with symptomatic severe or critical coronavirus disease 2019 (COVID-19) have been found to have an increased risk for preterm birth (PTB) [1,2,3], the association of the pandemic time period itself—involving societal disruptions and changes in healthcare utilization—with PTB is less clear. Some studies have reported a reduction in PTB [4,5,6,7] and others have found no change [8,9,10,11,12]. There is similar discordance among reports of a change in stillbirth [8, 13, 14]. Specifically, during the early months of the pandemic our team found that the only significant difference in birth outcomes during the pandemic period compared to matched months prior years was a decrease in spontaneous PTB (sPTB) among non-Hispanic White patients [15].
Identifying risk factors for PTB is complicated by its multifaceted and numerous etiologies. To aid in a deeper understanding of the sociodemographic, psychosocial, and clinical attributes associated with increased risk of PTB, analyses should, at least, be stratified by the two major phenotypes [16] of PTB, sPTB and medically indicated PTB (mPTB). Additionally, greater insight into risk profiles and the biological processes through which risk is conferred can be gleaned through the assessment of the gestational timing of exposure to a stressor and the risk of different PTB phenotypes [17]. Analyses that incorporate PTB phenotype and gestational timing of exposures may help elucidate factors that underpin the racial and ethnic disparity in the rate of PTB found in the U.S [18]. The COVID-19 pandemic has had a disproportionate impact on Black individuals in the U.S [19]. Black communities have experienced higher COVID-19 infection and mortality rates [20, 21] and have reported higher levels of pandemic-associated stress during pregnancy [22] compared to White communities, potentially widening existing gaps in adverse pregnancy outcomes.
Like other studies [5, 6, 8,9,10], the initial analysis in Philadelphia [15] only included four months of data and did not examine the effect of the duration of exposure to the pandemic. Therefore, the objective of this study was to further explore the relationship of duration of exposure to the pandemic with overall PTB, its phenotypes (sPTB, mPTB), gestational age sub-categories (extreme, very, moderate, and late PTB), and stillbirth utilizing a larger dataset. We included stillbirth in our analysis to ensure that if we detected lower PTB rates, it was not due to increases in stillbirth. Given the finding of reduced sPTB among non-Hispanic White patients in prior initial analyses [15], we examined changes in racial disparities in PTB in the pandemic compared to the pre-pandemic period and we hypothesized that with longer pandemic exposure reflected in more months of data we would detect an increase in racial disparities in birth outcomes during the pandemic compared to prior years.
Methods
We analyzed birth outcomes in GeoBirth, a retrospective cohort of all singleton births at the Hospital of the University of Pennsylvania (HUP) and Pennsylvania Hospital (PAH) in Philadelphia, PA. Within GeoBirth, each PTB (<37 weeks’ gestation) was manually reviewed and determined to be an sPTB (preterm labor, spontaneous rupture of membranes) or a mPTB (clinician initiated due to a maternal or fetal health condition) by two independent blinded reviewers. If there was discordance between the reviewers, a third party reviewed the chart and made the final determination. Since racial disparities have been shown to vary by severity of PTB [23], we also examined rate of extreme (20–<28 weeks’ gestation), very (28–<32 weeks’ gestation), moderate (32–<34 weeks’ gestation), or late (34–<37 weeks’ gestation) PTB. Gestational age was determined based on best obstetric clinical estimate. Stillbirth was defined as documentation of intrauterine fetal demise from the electronic health record (EHR) at ≥20 weeks’ gestation. We then replicated our findings using data from Yale New Haven Health System in New Haven, CT, for which the only difference was that the classification of sPTB was done through an automated EHR query. A PTB was classified as spontaneous if there was documentation of spontaneous preterm labor or preterm premature rupture of membranes. All other PTBs were considered mPTB. This study was approved by the University of Pennsylvania and Yale University Institutional Review Boards with waivers of informed consent.
We compared patients with births during the pandemic (March 10, 2020–December 31, 2020) to those with births during analogous months in the prior two years (2018 and 2019). We defined our cohort by restricting the pandemic period to patients with a last menstrual period (LMP) ≤20 weeks before the pandemic onset (March 10, 2020), and an estimated due date before December 17, 2020; we matched the pre-pandemic period by these constraints (Fig. 1). In this way, all patients’ pandemic exposure started on or before 20 weeks’ gestation. To examine whether the duration of exposure to the pandemic mattered, we split the cohort into two groups: those whose exposure to the pandemic began during their first trimester (<14 weeks’ gestation) and those whose exposure began after their first trimester (14–≤20 weeks’ gestation). We did not include patients with earlier LMPs (before October 22, 2019) because their exposure to the pandemic began later in pregnancy and some patients with the same LMP may have already had a PTB prior to the pandemic. For example, a patient with a LMP of October 20, 2019 who delivered preterm at 20 weeks on March 8, 2020 would not have been captured in the analysis because patients were identified from the birth records. Using this approach, only patients exposed to the pandemic and eligible for a PTB during the pandemic were included in the pandemic period cohort.
We used log-binomial regression models to estimate relative risks of overall PTB, sPTB, mPTB, extreme, very, moderate, and late PTB, as well as stillbirth during the pandemic period compared to the pre-pandemic period. Given their association with PTB in prior studies, we decided a priori to adjust for age, parity, pre-pregnancy body mass index (BMI), smoking, and insurance status; we used a missing indicator for patients without data for pre-pregnancy BMI or smoking status. In analyses stratified by race we combined extreme and very PTB due to small numbers. Additionally, we conducted a sensitivity analysis restricted to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) negative patients in the GeoBirth cohort to ensure that findings were not due to infection; universal SARS-CoV-2 screening upon hospital admission for childbirth was conducted with real-time reverse transcriptase–polymerase chain reaction testing beginning on April 1 and April 13, 2020, at HUP and PAH, respectively. Any positive test documented in the EHR up until the time of birth was counted. We tested for interactions of the pandemic period with duration of exposure and racial disparities using the ratio of relative risks [24]. Participant race and ethnicity were self-reported and categorized as Asian, Hispanic, multiple or unknown (grouped due to small numbers), non-Hispanic Black (Black), and non-Hispanic White (White). Analyses of GeoBirth data were conducted using R, version 4.0.2; code available at https://github.com/annemullin/covid_disparities_jperi.git. Analyses of Yale data were done using SAS 9.4, Cary, NC. Both analyses used a 2-tailed significance threshold of p < 0.05.
Results
Primary analyses in Philadelphia
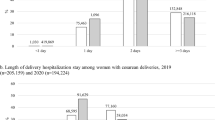
In the primary GeoBirth dataset from Philadelphia, there were 10,610 births (n = 7,163 in the pre-pandemic period and n = 3,447 during the pandemic). In the pandemic period, there were 2385 patients for whom the pandemic began prior to 14 weeks’ gestation and 1062 patients for whom the pandemic began between 14 and 20 weeks’ gestation. Patient characteristics were similar in the pre-pandemic and the pandemic periods (Table 1). The average age was 30.7 years old, 43.9% self-identified as Black, and 44.1% were publicly insured. We did not detect a significant difference in the PTB rate in the pre-pandemic (9.6%) and pandemic (9.1%) periods (p = 0.42) (Table 2). The PTB rates among patients exposed to the pandemic starting <14 weeks’ gestation (8.8%) and ≥14 weeks’ gestation (10.0%) were also similar to the pre-pandemic rate of 9.6% (p = 0.25 and p = 0.85, respectively). Multivariable adjustment did not affect the findings; there was no detectable difference in PTB during the pandemic among all patients exposed, or among the subgroups exposed to the pandemic <14 or ≥14 weeks’ gestation (Fig. 1). Additionally, there were no detectable differences in any of the subcategories of PTB examined including sPTB, mPTB, or in extreme, very, moderate, or late PTB. There were no detectable changes in stillbirth rates which were 6/1,000 before the pandemic and 4/1,000 in the pandemic period (p = 0.49).
When we examined whether exposure to the pandemic at <14 weeks’ or ≥14 weeks’ gestation had significant differences in the effect estimates of pandemic exposure with each PTB outcome, there was only one significant interaction. Patients exposed to the pandemic <14 weeks’ gestation had non-significantly decreased risk of extremely PTB (<28 weeks’) (aRR 0.60, 95% CI: 0.30–1.10) whereas those exposed ≥14 weeks’ gestation had non-significantly increased risk (aRR 1.77, 95% CI: 0.78–3.97) compared to pre-pandemic (interaction p = 0.04) (Fig. 2).
With respect to the association of pandemic exposure with racial disparities in outcomes, we performed two analyses. First, we examined outcomes within each racial stratum. The only significant difference detected during the pandemic was a decrease in very PTB (<32 weeks’ gestation) among White patients from 1.2% pre-pandemic to 0.5% during the pandemic (p = 0.04) (Table 3). There were no other significant differences. To analyze changes in disparities we calculated ratios of relative risk of each outcome separately among Black and a combined group of Asian, Hispanic, multiple or unknown patients compared to White patients. There were no significant differences. In sensitivity analyses of the GeoBirth cohort, we excluded the 139 patients who tested positive for SARS-CoV-2 up until the time of birth and results were similar (Supplemental Tables 4 & 5).
Replication analyses in New Haven
We performed an analogous set of analyses in the replication cohort from Yale New Haven Health System. There were 6194 births: 4173 pre-pandemic and 2021 during the pandemic (Supplemental Table 1). There were 1335 births among patients exposed to the pandemic <14 weeks’ gestation and 686 exposed ≥14 weeks’ and ≤20 weeks’ gestation. The Yale New Haven cohort differed from the GeoBirth Philadelphia cohort. A lower proportion of patients self-identified as Black at Yale (18.0%) compared with GeoBirth (43.9%). Rates of public insurance were slightly different as well (39.6% at Yale compared to 44.1% in GeoBirth). With respect to PTB outcomes, there were no changes during the pandemic compared to pre-pandemic at Yale in all patients (Supplemental Table 2). Furthermore, there were no detectable differences based on duration of exposure to the pandemic (interaction p-values all >0.05) (Fig. 2).
With respect to racial disparities, the reduction in very PTB (<32 weeks’ gestation) among White patients in Philadelphia was not observed in New Haven. In New Haven, in the pre-pandemic period, 0.9% of White patients had very PTB compared to 1.2% during the pandemic and in adjusted models, Black-White disparities were less pronounced during the pandemic compared to prior (Fig. 3). For example, in adjusted pre-pandemic models, Black patients were 59% (aRR 1.59, 95% CI: 1.18–2.15) more likely to have PTB, but only 5% more likely during the pandemic (aRR 1.05, 95% CI: 0.67–1.65) (interaction p = 0.05). The Black-White disparity in mPTB narrowed during the pandemic while the Asian, Hispanic, multiple, or unknown-White mPTB disparity widened. There was a significant narrowing of both Black-White and Asian, Hispanic, multiple, or unknown-White disparities in extreme and very PTB. However, there was a non-significant increase in the Black-White disparity in stillbirths (pre-pandemic aRR 2.02, 95% CI: 0.68–6.00 and pandemic aRR 3.74, 95% CI: 0.57–24.6) (interaction p = 0.12).
Discussion
In this study using data from two U.S. urban academic medical centers, we did not detect changes in PTB during the pandemic compared to matched months prior. Furthermore, when we stratified pregnant patients by pandemic exposure <14 weeks’ gestation and ≥14 weeks’ gestation, we did not observe changes in overall PTB compared to pre-pandemic. In the GeoBirth cohort in Philadelphia, we found that patients exposed to the pandemic earlier than 14 weeks’ gestation had decreased risk of extreme PTB compared to those exposed later in gestation. Since the results did not replicate in New Haven, this finding may be due to chance (type I error) since we did multiple tests and did not correct for multiple testing. Furthermore, there were very few extreme PTBs during the pandemic (n = 23). When we stratified by race, only PTB < 32 weeks’ gestation decreased among White patients in Philadelphia, which non-significantly widened the disparity in this outcome only–a trend that did not replicate in New Haven and may also be spurious in the setting of multiple comparisons. However, a post-hoc analysis of 2021 PTB < 32 weeks’ gestation revealed a drop in rates among Black patients compared to pre-pandemic, narrowing the disparity.
Changes in many aspects of life during the pandemic could have led to changes in health and birth outcomes, including but not limited to pollution exposure, occupational demands, physical activity, diet and nutrition, psychosocial stress and mental health status, substance use, sleep patterns, and infectious exposures. However, the extent to which individuals might have benefited from or been harmed by the pandemic could be a multitude of factors including socioeconomic strata, household composition, and employment status/type. These variations in life circumstances may contribute to the inconsistent findings with respect to pandemic-associated birth outcomes across the literature.
Our findings differ from European reports showing decreases in PTB [6, 25], but are consistent with a study from Ontario, Canada [13] and a study of extreme preterm infant NICU admissions across 17 countries [12]. U.S. reports have varied. U.S. birth certificate data show a subtle reduction in PTB rates to 10.09% in 2020 from 10.23% in 2019 [26]. With the exception of a sPTB reduction among White patients, the first four months of the pandemic did not reveal changes in birth outcomes in GeoBirth in Philadelphia [15]. One large study analyzed 838,489 singleton births from across the U.S. using data from Epic’s Cosmos research platform and did not find differences in overall rates of PTB and stillbirth, but could not assess changes in sPTB, mPTB, or specific gestational age windows [27]. In contrast, a population-based study of 296,934 births in Colorado observed decreased PTB rates during the pandemic, which was driven by a significant decline among White and Hispanic individuals [28]. Results were similar in an analysis of 49,845 births in Tennessee [5]. Neither study evaluated sPTB or mPTB separately. Finally, none of these published studies accounted for the timing of pandemic onset with respect to the duration of pandemic and pandemic-associated societal changes on birth outcomes.
The COVID-19 pandemic is not the only societal change that presented an opportunity to examine a natural experiment to elucidate drivers of PTB. There are several studies of other natural experiments (natural disasters, political events) and birth outcomes. A study in New York City demonstrated that among patients who were in their first trimesters during the World Trade Center attack on September 11, 2001, gestations were slightly shorter (−3.6 days), but the study did not report PTB rates [29]. Ahmed et al. assessed if exposure to an ice storm in Quebec increased the risk of PTB and if trimester at exposure modified this association [30]. No differences in the rate of PTB was observed between exposed and non-exposed individuals and no trimester-specific effect was observed. However, Auger et al. reported that there were higher singleton PTB rates in the “Triangle of Darkness” which was most affected by the storm, compared to other time periods as well as other less-affected areas of metropolitan Montréal [31]. Others examined the effect of exposure to an earthquake in New Zealand on PTB rates by comparing exposed patients carrying singleton pregnancies in their first trimester to a similar unexposed cohort [32]. Again, no effect on PTB was observed. However, using a Swedish cohort of over 2 million births, Class et al, found that those exposed to severe life stress, as defined by the death of a spouse or first degree relative, were significantly more likely to experience PTB and, those exposed in mid gestation (months five and six) were statistically significantly more at risk compared to those exposed in other gestational windows [33]. Finally, an interrupted time-series study examined PTB during the Muslim travel ban to the U.S. from September 2017 through August 2018 and showed 6.8% increased odds in PTB among individuals from countries affected by the ban [34]. Taken together, some natural experiments have shown associations between acute stressors and birth outcomes, and others have not.
One of the strengths of our study is the inclusion of timing of exposure to the pandemic. In our study, we carefully evaluated timing. For example, a person who gave birth at 41 weeks’ gestation in the first week of April 2020 in the U.S., had only a few weeks of exposure to pandemic life and none of those weeks could have resulted in a PTB because all exposure occurred after 37 weeks’ gestation. Dating pregnancy by LMP (or calculated LMP based on the best obstetric estimate of gestational age at birth) allows for concurrent comparison of individuals who are equally eligible to have various outcomes at different points during pregnancy. Other strengths of our study include consideration of the distinct phenotypes of PTB with respect to sPTB, mPTB, various gestational age categories, and stillbirth rates. Additionally, our primary cohort included a high proportion of births to Black people, a population disproportionately affected by COVID-19, and our analysis was replicated using an external dataset. Limitations of our study include the inability to analyze rates of spontaneous abortion due to insufficient data and the use of health system, as opposed to population-based data. It is possible that how patients chose a birth hospital changed during the pandemic which could alter center-specific PTB rates if the patient population changed between time periods. We were reassured that measured demographic characteristics were similar in the pre-pandemic and pandemic periods. Another limitation is the size of our dataset. Had our dataset been significantly larger it is plausible that the non-significant decrease in overall PTB rates between periods in GeoBirth (9.6% pre-pandemic to 9.1% pandemic) could have reached statistical significance. A post-hoc power analysis revealed that 39,900 births in the pandemic era and twice that number in the pre-pandemic era would have been necessary for this small difference in PTB to be statistically significant. However, given that our results did not replicate in New Haven, it is unlikely that there was a universal lowering of PTB rates during the pandemic. Additionally, the psychosocial stress caused by the pandemic may have longer-term effects that have yet to be measured. Finally, the SARS-CoV-2 positivity rate may have been lower during the study period than in more recent months and it is unknown whether findings would change in the more recent waves of the pandemic.
In conclusion, in a detailed analysis of PTB phenotypes and timing of pandemic exposure, we did not detect significant differences in PTB outcomes during the pandemic compared to matched months in prior years. The one exception was a reduction in very PTB (<32 weeks’ gestation) among White patients resulting in a non-significant widening of the Black-White disparity in this outcome in the Philadelphia-based GeoBirth cohort. This finding hints at the complexity of evaluating the effect of this natural experiment on rates of PTB. Although at first glance it may seem that exposure is universal, there is evidence that the negative consequences of the pandemic were not evenly distributed. Not surprisingly, vulnerable subgroups already living with fewer resources to absorb economic shocks were devastated by the pandemic. It is reasonable to assume that families with no option for telework and with no resources to manage childcare issues created by school closures suffered more than those with the flexibility to work from home and resources to secure creative options for their children [35]. Conversely, it is reasonable to assume that for some select groups the alterations in daily life caused by the pandemic may have been stress-reducing [36]. Thus, the varied findings across studies of pandemic-associated birth outcomes globally, nationally, regionally, and locally highlight the complexity and heterogeneity of environmental and sociostructural factors that contribute to maternal-child health.
Data availability
Upon request to, the PI of the cohort, Dr. Burris will discuss collaboration and sharing of deidentified data that will be contingent upon approval by all team members and institutional approval.
References
Pierce-Williams RAM, Burd J, Felder L, Khoury R, Bernstein PS, Avila K, et al. Clinical course of severe and critical coronavirus disease 2019 in hospitalized pregnancies: a United States cohort study. Am J Obstet Gynecol MFM. 2020;2:100134.
Dubey P, Reddy SY, Manuel S, Dwivedi AK. Maternal and neonatal characteristics and outcomes among COVID-19 infected women: An updated systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2020;252:490–501.
Metz TD, Clifton RG, Hughes BL, Sandoval G, Saade GR, Grobman WA, et al. Disease severity and perinatal outcomes of pregnant patients with Coronavirus disease 2019 (COVID-19). Obstet Gynecol. 2021;137:571–80.
Been JV, Burgos Ochoa L, Bertens LCM, Schoenmakers S, Steegers EAP, Reiss IKM. Impact of COVID-19 mitigation measures on the incidence of preterm birth: a national quasi-experimental study. Lancet Public Health. 2020;5:e604–11.
Harvey EM, McNeer E, McDonald MF, Shapiro-Mendoza CK, Dupont WD, Barfield W, et al. Association of preterm birth rate with COVID-19 statewide stay-at-home orders in Tennessee. JAMA Pediatr. 2021. https://doi.org/10.1001/jamapediatrics.2020.6512.
Hedermann G, Hedley PL, Bækvad-Hansen M, Hjalgrim H, Rostgaard K, Poorisrisak P, et al. Danish premature birth rates during the COVID-19 lockdown. Arch Dis Child—Fetal Neonatal Ed. 2021;106:93–5.
Yang J, D’Souza R, Kharrat A, Fell DB, Snelgrove JW, Murphy KE, et al. COVID-19 pandemic and population-level pregnancy and neonatal outcomes: a living systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2021;100:1756–70.
Khalil A, von Dadelszen P, Draycott T, Ugwumadu A, O’Brien P, Magee L. Change in the incidence of stillbirth and preterm delivery during the COVID-19 pandemic. JAMA. 2020;324:705–6.
Wood R, Sinnott C, Goldfarb I, Clapp M, McElrath T, Little S. Preterm birth during the Coronavirus Disease 2019 (COVID-19) pandemic in a large hospital system in the United States. Obstet Gynecol. 2021;137:403–4.
Janevic T, Glazer KB, Vieira L, Weber E, Stone J, Stern T, et al. Racial/ethnic disparities in very preterm birth and preterm birth before and during the COVID-19 pandemic. JAMA Netw Open. 2021;4:e211816.
Pasternak B, Neovius M, Söderling J, Ahlberg M, Norman M, Ludvigsson JF, et al. Preterm birth and stillbirth during the COVID-19 pandemic in Sweden: A Nationwide Cohort Study. Ann Intern Med. 2021;174:873–5.
Rasmussen MI, Hansen ML, Pichler G, Dempsey E, Pellicer A, EL-Khuffash A, et al. Extremely preterm infant admissions within the SafeBoosC-III Consortium during the COVID-19 lockdown. Front Pediatr. 2021; 9. https://www.frontiersin.org/article/10.3389/fped.2021.647880 (accessed 22 Jun 2022).
Shah PS, Ye XY, Yang J, Campitelli MA. Preterm birth and stillbirth rates during the COVID-19 pandemic: a population-based cohort study. CMAJ. 2021;193:E1164–72.
Curtis MD, Villani L, Polo A. Increase of stillbirth and decrease of late preterm infants during the COVID-19 pandemic lockdown. Arch Dis Child—Fetal Neonatal Ed. 2021;106:456.
Handley SC, Mullin AM, Elovitz MA, Gerson KD, Montoya-Williams D, Lorch SA, et al. Changes in preterm birth phenotypes and stillbirth at 2 Philadelphia hospitals during the SARS-CoV-2 Pandemic, March–June 2020. JAMA. 2021;325:87.
Henderson JJ, McWilliam OA, Newnham JP, Pennell CE. Preterm birth aetiology 2004–2008. Maternal factors associated with three phenotypes: spontaneous preterm labour, preterm pre-labour rupture of membranes and medically indicated preterm birth. J Matern Fetal Neonatal Med. 2012;25:642–7.
Zhu P, Tao F, Hao J, Sun Y, Jiang X. Prenatal life events stress: implications for preterm birth and infant birthweight. Am J Obstet Gynecol. 2010;203:34.e1–34.e8.
National Vital Statistics Reports Volume 70, Number 2, March 23 Births: Final Data for 2019. 2019; 51.
Millett GA, Jones AT, Benkeser D, Baral S, Mercer L, Beyrer C, et al. Assessing differential impacts of COVID-19 on black communities. Ann Epidemiol. 2020;47:37–44.
Garg S, Kim L, Whitaker M, O’Halloran A, Cummings C, Holstein R, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed Coronavirus Disease 2019—COVID-NET, 14 States, March 1–30, 2020. Morb Mortal Wkly Rep. 2020;69:458–64.
Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among black patients and white patients with Covid-19. N. Engl J Med. 2020;382:2534–43.
Preis H, Mahaffey B, Heiselman C, Lobel M. Vulnerability and resilience to pandemic-related stress among U.S. women pregnant at the start of the COVID-19 pandemic. Soc Sci Med. 2020;266:113348.
Egbe TI, Montoya-Williams D, Wallis K, Passarella M, Lorch SA. Risk of extreme, moderate, and late preterm birth by maternal race, ethnicity, and nativity. J Pediatr. 2022;240:24–30.e2.
Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326:219.
Einarsdóttir K, Swift EM, Zoega H. Changes in obstetric interventions and preterm birth during COVID-19: A nationwide study from Iceland. Acta Obstet Gynecol Scand. 2021;100:1924–30.
Hamilton B, Martin J, Osterman M, Births: Provisional Data for 2020. National Center for Health Statistics, 2021 https://doi.org/10.15620/cdc:104993.
Son M, Gallagher K, Lo JY, Lindgren E, Burris HH, Dysart K, et al. Coronavirus Disease 2019 (COVID-19) pandemic and pregnancy outcomes in a U.S. Population. Obstet Gynecol. 2021. https://doi.org/10.1097/AOG.0000000000004547.
Hwang SS, Weikel BW, Hannan KE, Bourque SL Impact of COVID-19 “Stay-At-Home” Orders on Preterm Birth in Colorado. J Pediatr 2021. https://doi.org/10.1016/j.jpeds.2021.10.046.
Lederman SA, Rauh V, Weiss L, Stein JL, Hoepner LA, Becker M, et al. The effects of the World Trade Center event on birth outcomes among term deliveries at three Lower Manhattan Hospitals. Environ Health Perspect. 2004;112:1772–8.
Ahmed A, King S, Elgbeili G, Laplante DP, Yang S. Effects of maternal exposure to acute stress on birth outcomes: a quasi-experiment study. J Dev Orig Health Dis. 2021;112:1772–8.
Auger N, Kuehne E, Goneau M, Daniel M. Preterm birth during an extreme weather event in Québec, Canada: a ‘natural experiment’. Matern Child Health J. 2011;15:1088–96.
Hawkins G, Gullam J, Belluscio L. The effect of a major earthquake experienced during the first trimester of pregnancy on the risk of preterm birth. Aust N. Z J Obstet Gynaecol. 2019;59:82–88.
Class QA, Lichtenstein P, Långström N, D’Onofrio BM. Timing of prenatal maternal exposure to severe life events and adverse pregnancy outcomes: A population study of 2.6 million pregnancies. Psychosom Med. 2011;73:234–41.
Samari G, Catalano R, Alcalá HE, Gemmill A. The Muslim Ban and preterm birth: Analysis of U.S. vital statistics data from 2009 to 2018. Soc Sci Med 1982. 2020;265:113544.
Angelucci M, Angrisani M, Bennett DM, Kapteyn A, Schaner SG, Remote work and the heterogeneous impact of COVID-19 on employment and health. National Bureau of Economic Research, 2020 https://doi.org/10.3386/w27749.
Birimoglu Okuyan C, Begen MA. Working from home during the COVID-19 pandemic, its effects on health, and recommendations: The pandemic and beyond. Perspect Psychiatr Care. 2022;58:173–9.
Funding
This study was funded by Highmark Blue Cross Blue Shield Delaware’s donor-advised fund, BluePrints for the Community, and Independence Blue Cross. The Department of Pediatrics at the Children’s Hospital of Philadelphia supported the time and effort of Drs. Burris, Montoya-Williams, and Handley. Dr. Montoya-Williams reports receiving research funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (K23HD102526).
Author information
Authors and Affiliations
Contributions
AMM designed and performed the analysis in Philadelphia and wrote the manuscript. SCH, MAE, SAL, DM-W, NY, KD, MS, JG, and JFC co-designed the study, reviewed the results, and critically edited the manuscript. LL performed the analysis in New Haven and critically edited the manuscript. EJM designed study variables and critically reviewed the manuscript. HHB mentored AMM through all aspects of the study and critically reviewed the manuscript. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was performed in accordance with the Declaration of Helsinki and approved by the University of Pennsylvania and Yale University Institutional Review Boards with waivers of informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mullin, A.M., Handley, S.C., Lundsberg, L. et al. Changes in preterm birth during the COVID-19 pandemic by duration of exposure and race and ethnicity. J Perinatol 42, 1346–1352 (2022). https://doi.org/10.1038/s41372-022-01488-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-022-01488-1