Abstract
Objective
To determine if treatment with a 5-HT3 antagonist (ondansetron) reduces need for opioid therapy in infants at risk for neonatal opioid withdrawal syndrome (NOWS).
Study design
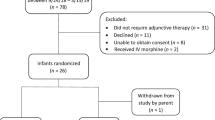
A multicenter, randomized, placebo controlled, double blind clinical trial of ninety (90) infants. The intervention arms were intravenous ondansetron or placebo during labor followed by a daily dose of ondansetron or placebo in infants for five days.
Results
Twenty-two (49%) ondansetron-treated and 26 (63%) placebo-treated infants required pharmacologic treatment (p > 0.05). The Finnegan score was lower in the ondansetron-treated group (4.6 vs. 5.6, p = 0.02). A non-significant trend was noted for the duration of hospitalization. There was no difference in need for phenobarbital or clonidine therapy, or total dose of morphine in the first 15 days of NOWS treatment.
Conclusions
Ondansetron treatment reduced the severity of NOWS symptoms; and there was an indication that it could reduce the length of stay.
Clinical Trial Registration
Clinicaltrials.gov NCT01965704
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Disher T, Gullickson C, Singh B, Cameron C, Boulos L, Beaubien L, et al. Pharmacological treatments for neonatal abstinence syndrome: a systematic review and network meta-analysis. JAMA Pediatr. 2019;173:234–43.
Patrick SW, Barfield WD, Poindexter BB, Committee on fetus and newborn, committee on substance use and prevention. neonatal opioid withdrawal syndrome. Pediatrics 2020 Nov;146: https://doi.org/10.1542/peds.2020-029074.
Peltz G, Sudhof TC. The neurobiology of opioid addiction and the potential for prevention strategies. JAMA. 2018;319:2071–2.
Chu LF, Liang DY, Li X, Sahbaie P, D’arcy N, Liao G, et al. From mouse to man: the 5-HT3 receptor modulates physical dependence on opioid narcotics. Pharmacogenet Genomics. 2009;19:193–205.
Erlendson MJ, D’Arcy N, Encisco EM, Yu JJ, Rincon-Cruz L, Peltz G, et al. Palonosetron and hydroxyzine pre-treatment reduces the objective signs of experimentally-induced acute opioid withdrawal in humans: a double-blinded, randomized, placebo-controlled crossover study. Am J Drug Alcohol Abus. 2017;43:78–86.
Elkomy MH, Sultan P, Carvalho B, Peltz G, Wu M, Clavijo C, et al. Ondansetron pharmacokinetics in pregnant women and neonates: towards a new treatment for neonatal abstinence syndrome. Clin Pharm Ther. 2015;97:167–76.
Jones HE, O’Grady KE, Kaltenbach K. Reconsidering retrospective review of neurodevelopmental outcomes in infants treated for neonatal abstinence syndrome. J Perinatol. 2018;38:1280–1.
Merhar SL, McAllister JM, Wedig-Stevie KE, Klein AC, Meinzen-Derr J, Poindexter BB. Retrospective review of neurodevelopmental outcomes in infants treated for neonatal abstinence syndrome. J Perinatol. 2018;38:587–92.
Oei JL, Melhuish E, Uebel H, Azzam N, Breen C, Burns L, et al. Neonatal Abstinence Syndrome and High School Performance. Pediatrics 2017;139: https://doi.org/10.1542/peds.2016-2651.
Ornoy A. The impact of intrauterine exposure versus postnatal environment in neurodevelopmental toxicity: long-term neurobehavioral studies in children at risk for developmental disorders. Toxicol Lett. 2003;140-141:171–81.
Hunt RW, Tzioumi D, Collins E, Jeffery HE. Adverse neurodevelopmental outcome of infants exposed to opiate in-utero. Early Hum Dev. 2008;84:29–35.
Moore JN, Gastonguay MR, Ng CM, Adeniyi-Jones SC, Moody DE, Fang WB, et al. The pharmacokinetics and pharmacodynamics of buprenorphine in neonatal abstinence syndrome. Clin Pharm Ther. 2018;103:1029–37.
Tyers MB, Freeman AJ. Mechanism of the anti-emetic activity of 5-HT3 receptor antagonists. Oncology. 1992;49:263–8.
Tyers MB. Site(s) and mechanisms of the anti-emetic action of 5-HT3 receptor antagonists: a discussion of Professor Naylor’s paper. Br J Cancer Suppl. 1992;19:S12–3.
Portenoy RK, Thomas J, Moehl Boatwright ML, Tran D, Galasso FL, Stambler N, et al. Subcutaneous methylnaltrexone for the treatment of opioid-induced constipation in patients with advanced illness: a double-blind, randomized, parallel group, dose-ranging study. J Pain Symptom Manag. 2008;35:458–68.
Thomas J, Karver S, Cooney GA, Chamberlain BH, Watt CK, Slatkin NE, et al. Methylnaltrexone for opioid-induced constipation in advanced illness. N Engl J Med. 2008;358:2332–43.
Simpson KH, Murphy P, Colthup PV, Whelan P. Concentration of ondansetron in cerebrospinal fluid following oral dosing in volunteers. Psychopharmacol (Berl). 1992;109:497–8.
Acknowledgements
We acknowledge the work of the following site principal investigators who assisted in the conduct of the study. Lori A. Devlin, MD (Univ. of Louisville), Ramasubbareddy Dhanireddy, MD (University of Tennessee Health Science Center), Ronald S. Cohen, MD (Lucile Packard Children’s Hospital), Camille M. Fung, MD (University of Utah), Juan E. Vargas, MD (University of California, San Francisco General Hospital). This trial was supported by National Institute of Drugs of Abuse (NIDA) R01 HD070795-06A1. Gary Petlz and Manhong Wu were also supported by a NIH/NIDA award 5U01DA04439902. The other authors received no additional funding. The NIH had no role in the design and conduct of the study.
Participating sites (number of subjects enrolled)
Thomas Jefferson University (42)
Johns Hopkins Bayview Medical Center (31)
Santa Clara Valley Medical Center (13)
University of Louisville (4)
University of Tennessee Health Science Center (3)
Lucile Packard Children’s Hospital (2)
University of Utah (2)
University of California, San Francisco General Hospital (1)
Author information
Authors and Affiliations
Contributions
GP conceptualized and designed the study, carried out the initial analyses, assisted with the initial draft, and reviewed and revised the manuscript. WKK drafted the initial manuscript, collected data, carried out the initial analyses, and reviewed and revised the manuscript. MW conducted statistical analysis, generated figures and tables, assisted with the initial draft, and reviewed and revised the manuscript. LMJ and SA-J collected data, contributed to the initial analyses, and reviewed and revised the manuscript. DD and SS, and CC coordinated and supervised data collection and review, contributed to the analyses, and reviewed and revised the manuscript. MW developed and conducted the analytical assay, and reviewed and revised the manuscript. BG, PJ, CA, and SK collected data, and reviewed and revised the manuscript
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Peltz, G., Jansson, L.M., Adeniyi-Jones, S. et al. Ondansetron to reduce neonatal opioid withdrawal severity a randomized clinical trial. J Perinatol 43, 271–276 (2023). https://doi.org/10.1038/s41372-022-01487-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-022-01487-2