Abstract
Objective
Describe 1-month outcomes among newborns of persons with perinatal COVID-19.
Study design
Prospective observational study of pregnant persons who tested positive for SARS-CoV-2 between 14 days before and 3 days after delivery and their newborns, from 3/2020 to 3/2021 at two urban high-risk academic hospitals. Phone interviews were conducted to determine 1-month newborn outcomes.
Results
Among 9748 pregnant persons, 209 (2.1%) tested positive for perinatal SARS-CoV-2. Symptomatically infected persons were more likely to have a preterm delivery due to worsening maternal condition and their newborns were more likely to test positive for SARS-CoV-2 compared with asymptomatic persons. Six of 191 (3.1%) infants tested were positive for SARS-CoV-2; none had attributable illness before discharge. Of 169 eligible families, 132 (78.1%) participated in post-discharge interviews; none reported their newborn tested positive for SARS-CoV-2 by 1 month of age.
Conclusion
Symptomatic perinatal COVID-19 had a substantial effect on maternal health but no apparent short-term effect on newborns.
Similar content being viewed by others
Introduction
During the early months of the coronavirus disease 2019 (COVID-19) pandemic, little information was available to guide management of perinatal COVID-19. Initial reports from China suggested that perinatal transmission of novel severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) was uncommon but could occur [1,2,3,4]. These reports described a practice of routine cesarean delivery for infected persons with immediate maternal-infant separation and isolation [5]. Based upon the limited early data from China, and knowledge of how other respiratory viruses, particularly influenza, affect pregnant people and newborns, the American Academy of Pediatrics (AAP) provided preliminary guidance in April 2020 with conservative initial recommendations for management of perinatal COVID-19 [6]. As the pandemic continues, our understanding of how the virus affects pregnant people and newborns has evolved. Accordingly, AAP guidance has been serially revised in part to reflect evidence that the risk of neonatal acquisition of SARS-CoV-2 infection in the hospital is low [5].
In utero fetal infection from pregnant mothers with SARS-CoV-2 infection appears rare [7,8,9]. Maternal/newborn transmission during and immediately after delivery seems to occur with greater frequency, and risk may be decreased with infection prevention practices. Although most infected newborns are asymptomatic or mildly symptomatic, there are case reports of severe neonatal SARS-CoV-2 infection, including cardiorespiratory failure and death [9, 10]. There is conflicting evidence regarding the intrapartum and postpartum factors that influence the infection risk to newborns, and little data on newborn infection risk after birth hospital discharge [11]. In this study, we sought to describe the relationship between perinatal COVID-19 and maternal and newborn health outcomes, and our local incidence of newborn SARS-CoV-2 infection during the delivery admission and within 1 month after birth, over a 13-month period at two urban, high-risk birth hospitals.
Methods
Study setting and population
This was a prospective observational study of pregnant persons who presented for delivery at any gestational age during March 1, 2020, and March 31, 2021, at two high-risk, academic birth hospitals in Philadelphia, Pennsylvania. Combined, these two centers provide care for approximately 9,000 births annually, accounting for ~50% of births in the city of Philadelphia. Both centers instituted SARS-CoV-2 testing of nasopharyngeal swabs by reverse transcription polymerase chain reaction (RT-PCR) for parturient persons in March 2020 and implemented universal testing on admission in April 2020. As of June 2020, patients with documented infection during pregnancy were not retested if >28 days had passed since first test. As of August 2020, this time frame was changed to >20 days. Newborns of test-positive mothers were tested by nasopharyngeal swabs at 24 and 48 h after birth, with continued daily testing through discharge if initial testing was positive or for other reasons at the discretion of the clinician. Local guidelines for infection prevention practices and maternal/newborn separation changed over time during the study period, in accordance with AAP recommendations. Maternal/newborn dyad rooming-in was utilized as of June 2020, unless maternal or newborn severity of illness precluded this care model or unless the mother preferred temporary separation. When rooming-in, mothers were encouraged to use masks and hand hygiene when in contact with their newborn. Throughout the study period, all clinicians caring for newborns of RT-PCR-positive mothers utilized appropriate infection prevention and control practices and personal protective equipment. The Institutional Review Board at the University of Pennsylvania approved this study. The activity was reviewed by the US Centers for Disease Control and Prevention (CDC) and was conducted consistent with applicable federal law and CDC policy (45 C.F.R. part 46, 21 C.F.R. part 56; 42 U.S.C. Sect. 241(d); 5 U.S.C. Sect. 552a; 44 U.S.C. Sect. Sect. 3501 et seq.).
Pregnant persons who had a positive SARS-CoV-2 RT-PCR test result performed within the health system within 14 days before delivery, those identified as positive by routine obstetric testing on admission for delivery, and those identified as positive by symptom-indicated testing through 3 days after delivery, and their newborns, were included. Demographic and clinical data were extracted from the electronic medical record. A subset of de-identified data collected for this study were also submitted to the AAP Section on Neonatal Perinatal Medicine’s National Registry for Surveillance and Epidemiology of Perinatal COVID-19 Infection (NPC-19) [12]. Symptomatic maternal infection was defined as upper respiratory infection symptoms (cough, nasal congestion, sinus congestion, sore throat), lower respiratory infection symptoms (respiratory distress, cough, wheezing), fever, gastrointestinal symptoms (vomiting, diarrhea, nausea), myalgia, fatigue, anosmia, and/or ageusia. Maternal symptom onset during the perinatal period was defined as symptom onset between 14 days before and 3 days after delivery, and was determined based on maternal condition on admission (i.e., symptomatic versus asymptomatic) as well as interval in days between date of first maternal symptoms consistent with COVID-19 and date of delivery. Those without symptoms were either asymptomatic during the perinatal period or never symptomatic. Vaccination data was not collected as the majority of pregnancies were completed prior to widespread availability of COVID-19 vaccines.
Post-discharge follow-up
Families were contacted for structured phone interviews after birth hospital discharge. Exclusions for post-discharge interviews included cases of intrauterine fetal demise, newborn in-hospital death, neonatal intensive care unit (NICU) stay >7 days, primary language other than English or Spanish, or newborn testing positive for SARS-CoV-2 during the birth hospitalization. The interview questions focused on care of the newborn in the 1 month after birth hospital discharge. We attempted to contact each family up to three times on different days; if there was no answer on the third call, a message was left when possible, requesting a call back to the study team. Families were asked to provide verbal consent for the interview after description of the study. A research coordinator fluent in Spanish interviewed families identified as primary Spanish speaking during the birth hospitalization.
Statistical analysis
We examined maternal and neonatal demographic and clinical characteristics, medical interventions, and disposition, comparing pregnant persons with a positive PCR test result for SARS-CoV-2 with and without symptoms in the perinatal period. Standard descriptive and comparative statistics were performed. We first used Folded F’s tests and Wilk–Shapiro tests to assess equal variance and normality of the continuous variables, respectively. Given that none of the continuous variables had a normal distribution, we used the Kruskal–Wallis test to compare medians. We used Chi-Square tests for categorical variables, and Fisher’s Exact tests for variables with less than 5 occurrences. SAS 9.4 (Cary, North Carolina) was used for statistical analyses and two-sided P values < 0.05 were considered statistically significant.
Results
Study cohort
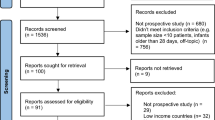
During the 13-month study period, there were 9748 pregnant persons who delivered 9925 infants at the two centers. Of these, 215 pregnant persons (2.2%) had a positive SARS-CoV-2 PCR test in the perinatal period; 209 (97.2%) had available data and were included in the analysis (Fig. 1). There were 214 newborn infants included in the analysis, including 6 sets of twins and 1 set of quadruplets.
Maternal characteristics and outcomes during birth hospitalization
Of the 209 pregnant persons who tested positive for SARS-CoV-2, 42 (20.1%) were symptomatic during the perinatal period and 167 (79.9%) were not. Of the 167 persons without symptoms in the perinatal period, 6 (3.6%) reported symptoms more than 14 days before delivery. Symptomatic persons were older and had longer duration between admission and delivery (Table 1). Median duration between onset of symptoms and delivery in the symptomatic group was 7 days (IQR 2, 9). Eight patients (3.8%) required hospitalization for SARS-COV-2 infection-related care in the 14 days prior to delivery. Symptomatic persons were more frequently administered COVID-specific medical treatment and had longer duration of hospitalization (Table 2). Supplemental oxygen was required for 7 (16.7%) of symptomatic persons and 2 (4.8%) required mechanical ventilation (Table 2). Pregnant persons with perinatal symptoms of COVID-19 more frequently underwent induction of labor and/or cesarean delivery due to worsening maternal condition (as reported by clinician) related to COVID-19 (10 (23.8%) vs. 0 (0.0%); P < 0.001) compared to asymptomatic persons (Table 1). None of the infected persons were transferred or died during the study period; all were discharged home.
Newborn characteristics and outcomes during birth hospitalization
Among the 209 pregnant persons with perinatal COVID-19, there were four singleton intrauterine fetal demises (at 15, 20, 35, and 39 weeks’ gestational age), and three singleton infants (born at 18, 20, and 27 weeks’ gestational age) who died in the delivery room or in the first day after birth (Fig. 1). Only one fetal death occurred among symptomatic mothers (born at 18 weeks’ gestation with death in the delivery room). None of the cases of fetal or infant deaths were tested for SARS-CoV-2.
Surviving newborns of mothers who were symptomatic in the perinatal period had lower gestational ages compared to mothers who were asymptomatic, but had similar birth weights (Table 1). No infants in either group received chest compressions and/or epinephrine in the delivery room, and intubation in the delivery room was similar between groups (Table 2). Proportions of infants admitted to the NICU at any time and duration of infant hospitalization were similar between the symptomatic and asymptomatic groups, overall, and when stratified by preterm status (Table 2). Potential signs of COVID-19 (fever, vomiting/diarrhea, respiratory distress) occurred more frequently among infants whose mothers were symptomatic compared to those whose mothers were asymptomatic during the perinatal period (9 (20.5%) vs. 15 (8.8%); p = 0.056). Overall, out of the 214 liveborn infants who survived, 91/212 (42.9%) were separated from their mothers at birth, and the rate of separation did not differ between symptomatic and asymptomatic mothers. Maternal/newborn separation during delivery hospitalization was significantly higher during March–August 2020 (73/119; 61.3 %) than September 2020–March 2021 (18/ 93; 19.4%) (P < 0.001) (Table 2).
Perinatal transmission of SARS-CoV-2 infection to newborns occurred more frequently when mothers were symptomatic in the perinatal period (Table 1, p = 0.017). Twenty-three infants were not tested for SARS-CoV-2 due to death, parent refusal, or other reasons. Of the 191 infants tested for SARS-CoV-2, six (3.1%) had a positive SARS-CoV-2 PCR test during the newborn hospitalization; four were born to mothers with perinatal symptoms (4/40; 10.0%) and two were born to mothers without perinatal symptoms (2/151; 1.3%) (Table 1). One infant tested positive for SARS-CoV-2 at 24 and 48 h and the other five infants tested negative for SARS-CoV-2 at 24 h and positive at 48 h (two of the three infants tested at 72 h remained positive) (Table 1). Only one of the infants who tested positive for SARS-Cov-2 had been separated from the mother at birth; this infant tested negative at 24 h and positive at 48 h. None of the infected infants developed significant illness that was attributed to the virus during the birth hospitalization as determined by the clinical care team.
Outcomes after birth hospital discharge
Of the 169 postpartum persons (of 174 infants given multiple births) eligible for post-discharge phone interview, 132 (78.1%) families (of 135 infants) were successfully contacted and agreed to participate in the phone survey (Fig. 1 and Table 3). Two newborns were readmitted to a hospital and an additional six were evaluated in an emergency department or an urgent care facility in the first month after discharge; no readmissions or evaluations were related to COVID-19. Sixteen of 135 (11.9%) infants were reported to have been retested for SARS-CoV-2 at the first routine pediatric visit and another 12/135 (8.9%) were retested at any other time between discharge and the phone interview. No infants were reported to have tested positive after discharge. Thirty-five of 131 (26.7%) families indicated that another household member tested positive for SARS-CoV-2 (Table 3); of these 23 (65.7%) tested positive before, 3 (8.6%) during, and 8 (22.9%) after maternal hospitalization (1 case had unknown timing).
Discussion
In this observational study of perinatal COVID-19 conducted at two high-risk, urban, academic birth hospitals, we found that symptomatic perinatal disease substantially affected maternal health (e.g., oxygen requirement, medically indicated preterm delivery). However, newborn outcomes did not differ by maternal symptom status. In the setting of infection prevention efforts after birth, only 3% of newborns acquired SARS-CoV-2 infection before hospital discharge, primarily (but not exclusively) among those born to mothers with symptomatic infection. Newborn infection within the first month after discharge was not reported.
Several findings in this study are aligned with other national and international reports addressing pregnant persons with SARS-CoV-2 infection near the time of labor and delivery. We found that pregnant persons with symptomatic SARS-CoV-2 infection near the time of delivery are more likely to require hospitalization for COVID-19 care and to undergo induction and/or cesarean delivery due to worsening infectious condition compared to those who tested positive but did not have symptoms in the perinatal period. Multiple reports now find that pregnant people are at higher risk for severe COVID-19 including death and adverse pregnancy and neonatal outcomes, but the effect of the timing and severity of infection on maternal outcome is less clear [13,14,15]. A systematic review and meta-analysis including ten studies assessed differences in outcomes among symptomatic versus asymptomatic COVID-19-infected pregnant people [16]. The pooled results suggested that symptomatic pregnant people were more likely to have a cesarean-section delivery, preterm delivery, and to require intensive care and mechanical ventilation [16]. The prospective, multinational INTERCOVID study also evaluated differences in outcomes between infected pregnant persons with and without symptoms and found that asymptomatic persons with a positive SARS-CoV-2 test result had outcomes more similar to persons who were presumed not to be infected with SARS-CoV-2, but this study did not account for timing of maternal infection during pregnancy [15]. Our study focuses on strictly defined perinatal infection and suggests that adverse maternal outcomes occur more commonly among infected pregnant persons in the setting of perinatal symptomatology. These findings support current recommendations for COVID-19 vaccination during pregnancy to prevent severe symptomatic disease [17].
We observed that perinatal transmission of SARS-CoV-2 occurred at a relatively low rate (3%) although infection did occur more frequently among newborns of pregnant persons with symptoms in the perinatal period. None of the infected infants developed significant illness during the birth hospitalization that was attributed to the virus, and adverse outcomes reported (e.g., NICU admission and need for respiratory support) occurred at frequencies that would be expected based on gestational age [18]. Additionally, there was no difference among neonates born to people with symptomatic perinatal infection compared with those with no symptoms. The INTERCOVID study found that 12.1% of infants born to test-positive mothers tested positive 24–48 h after birth; notably, this study included 18 countries with variable models of perinatal healthcare [15]. In contrast, a strength of our study is that newborn care was consistently directed by written guidelines, postnatal testing was done consistently per policy, and we utilized a limited number of validated test platforms in our hospital laboratories. In a systematic review of perinatal transmission of SARS-CoV-2 that included 49 studies of 655 pregnant persons and 666 infants, only 4% of infants tested positive after birth [19]. A statewide analysis of centers in Massachusetts found a 2.2% transmission rate, and a study from a large medical center in New York City found a 2.0% transmission rate, both of which are consistent with our results [20, 21]. The reasons for the low rate of maternal/newborn postnatal transmission have been debated. While we were not able to assess for possible in utero transmission, 5/6 of the test-positive newborns tested negative at 24 h and positive at 48 h, and importantly we observed that perinatal transmission did occur more frequently among newborns of perinatally-symptomatic pregnant persons. We speculate that perinatal transmission was likely due to postnatal acquisition (i.e., exposure to maternal secretions in the postpartum setting) from contagious mothers. The relatively low rates of newborn infection observed in studies to date may simply be due to the fact that universal PCR testing on obstetric hospital admission identifies many asymptomatic and not acutely contagious pregnant persons. Neonatal infection did occur, supporting international and national guidelines which advocate for infection prevention measures in those postpartum settings, when feasible. Consistent with other studies, we did not find a direct effect of maternal or neonatal SARS-CoV-2 infection on short-term newborn health during the birth hospitalization period [22, 23]. Although much has been learned in the past 2 years regarding how long persons are contagious when infected with SARS-CoV-2, less is known about the short- and long- term effects (if any) of infection in the immediate neonatal period. Given current evidence, including the findings of our study, we suggest that routine separation of asymptomatic parturient patients who test positive for SARS-CoV-2 on admission for delivery and their newborns is not warranted. Rather, shared decision-making should include an assessment of the likelihood that the patient is acutely infected and contagious to the newborn. Such decision-making can include education on the potential duration of contagion, the value of effective infection prevention efforts, and the apparent lack of short-term effects on the healthy term newborn.
The results of our post-discharge telephone interviews are similarly reassuring. No family reported their newborns to have tested positive for the virus by 1-month after discharge, despite a quarter of families reporting another household contact had also tested positive for SARS-CoV-2. This may be due to the protective role of maternal post-infection antibodies transferred transplacentally or in breast milk and/or the lower likelihood of severe disease in neonates that results in less frequent testing [24, 25]. The Massachusetts study also assessed outcomes for 59% of their cohort at 1 month, relying on electronic medical record review, and found only one case where an infant tested positive for the virus [20]. A study from New York City early in the pandemic found that no infants born to infected mothers had tested positive for SARS-COV-2 between day 3 and 25 after birth [21]. While use of infection prevention measures to protect newborns from the risk of infection should be in place in the immediate perinatal period, our findings, together with existing literature, provide further evidence that the benefits of maternal contact likely outweigh risks of postnatal infection.
The strengths of our study include a large cohort from two urban academic centers including births over a 13-month period. Telephone follow-up was performed in both English and Spanish; the structured interview format may reduce bias introduced from electronic medical record-based follow-up, given that infants may not receive follow-up care in the same health system where they are born. Our study does have limitations: We included only centers in one health system with strongly aligned care policies and, therefore, our results may not be generalizable to other settings. While this study included systematic testing of infants at 48 h of life, testing beyond the birth hospitalization was nonsystematic and based on clinical care. While we assessed SARS-CoV-2 infant infection in the first month after discharge, we could not assess longer-term or indirect effects of exposure to the virus in utero, nor could we measure the dynamics of maternally derived antibodies for infant protection. As data informing duration of infectiousness evolved during the first months of the pandemic, our local policies on maternal testing evolved and were not entirely consistent during the study period. The majority of pregnancies in this study were completed prior to the widespread availability of COVID-19 vaccines and we were not able to assess the impact of maternal vaccination. The results of our phone follow-up were subject to recall bias as follow-up calls occurred up to one year past birth in some cases. Finally, we cannot generalize our findings to infections due to COVID-19 variants identified after the study period.
Conclusions
In this observational study, while symptomatic perinatal COVID-19 infection affected maternal health and delivery, there was no clear effect on neonatal health after birth. Perinatal SARS-CoV-2 transmission to neonates was more likely when the mother was symptomatic in the perinatal period, and infants who tested positive for SARS-CoV-2 did not develop attributable clinical illness. Infant infection within the first month after discharge was not documented. Our findings support current recommendations for maternal vaccination during pregnancy to prevent maternal morbidity. For pregnant persons who test positive for SARS-CoV-2 around the time of delivery, the benefits of maternal contact likely outweigh the risks of neonatal infection, although longer-term and indirect consequences of infection during pregnancy have yet to be determined.
Disclaimer
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention (CDC).
Data availability
The data reported in this study are available to readers upon reasonable request.
References
Dong L, Tian J, He S, Zhu C, Wang J, Liu C, et al. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA. 2020;323:1846–8.
Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–15.
Zhu H, Wang L, Fang C, Peng S, Zhang L, Chang G, et al. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020;1:51–60.
Zeng L, Xia S, Yuan W, Yan K, Xiao F, Shao J, et al. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr. 2020. https://doi.org/10.1001/jamapediatrics.2020.0878.
Flannery DD, Puopolo KM. Perinatal COVID-19: guideline development, implementation, and challenges. Curr Opin Pediatr. 2021;33:188–94.
Wyckoff AS. AAP issues guidance on infants born to mothers with suspected or confirmed COVID-19 | AAP News | American Academy of Pediatrics. AAP News. 2020. https://publications.aap.org/aapnews/news/6713. Accessed 17 May 2022.
Ciapponi A, Bardach A, Comandé D, Berrueta M, Argento FJ, Cairoli FR, et al. COVID-19 and pregnancy: An umbrella review of clinical presentation, vertical transmission, and maternal and perinatal outcomes. PLoS ONE. 2021;16. https://doi.org/10.1371/JOURNAL.PONE.0253974.
Kollikonda S, Chavan M, Cao C, Yao M, Hackett L, Karnati S. Transmission of severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) through infant feeding and early care practices: a systematic review. J Neonatal Perinatal Med. 2022;15:209–17.
Molloy EJ, Lavizzari A, Klingenberg C, Profit J, Zupancic JAF, Davis AS, et al. Neonates in the COVID-19 pandemic. Pediatr Res. 2021;89:1038–40.
Shaiba LA, Altirkawi K, Hadid A, Alsubaie S, Alharbi O, Alkhalaf H, et al. COVID-19 disease in infants less than 90 days: case series. Front Pediatr. 2021;9:674899.
Briana D, Syridou G, Papaevangelou V. Perinatal COVID-19. Pediatr Infect Dis J. 2021;40:e504.
NPC-19 Registry. https://my.visme.co/view/ojq9qq8e-npc-19-registry. Accessed 16 Jan 2022.
Zambrano LD, Ellington S, Strid P, Galang RR, Oduyebo T, Tong VT, et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–October 3, 2020. MMWR. 2020;69:1641–7.
Allotey J, Stallings E, Bonet M, Yap M, Chatterjee S, Kew T, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:3320.
Villar J, Ariff S, Gunier RB, Thiruvengadam R, Rauch S, Kholin A, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr. 2021;175:817–26.
Khan DSA, Hamid L-R, Ali A, Salam RA, Zuberi N, Lassi ZS, et al. Differences in pregnancy and perinatal outcomes among symptomatic versus asymptomatic COVID-19-infected pregnant women: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2021;21:1–14.
Centers for Disease Control and Prevention. COVID-19 Vaccines While Pregnant or Breastfeeding 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html.
United States Department of Health and Human Services (US DHHS), Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS), Division of Vital Statistics, Natality public-use data 2016–2020, on CDC WONDER Online Database, October 2021. http://wonder.cdc.gov/natality-expanded-current.html. Accessed 16 Mar 2022.
Walker KF, O’Donoghue K, Grace N, Dorling J, Comeau JL, Li W, et al. Maternal transmission of SARS‐COV‐2 to the neonate, and possible routes for such transmission: a systematic review and critical analysis. BJOG An Int J Obstet Gynaecol. 2020:1471–0528. https://doi.org/10.1111/1471-0528.16362.
Angelidou A, Sullivan K, Melvin PR, Shui JE, Goldfarb IT, Bartolome R, et al. Association of maternal perinatal SARS-CoV-2 infection with neonatal outcomes during the COVID-19 pandemic in Massachusetts. JAMA Netw Open. 2021;4:e217523.
Dumitriu D, Emeruwa UN, Hanft E, Liao GV, Ludwig E, Walzer L, et al. Outcomes of neonates born to mothers with severe acute respiratory syndrome coronavirus 2 infection at a large medical center in New York City editorial supplemental content. JAMA Pediatr. 2020. https://doi.org/10.1001/jamapediatrics.2020.4298.
Brito I, Sousa R, Sanches B, Franco J, Marcelino S, Costa A. Rooming-in, breastfeeding and neonatal follow-up of infants born to mothers with COVID-19. Acta Med Port. 2021;34:507–16.
Lucovnik M, Druskovic M, Vidmar Simic M, Verdenik I, Mesaric V, Kosir R, et al. Perinatal outcomes in women with severe acute respiratory syndrome coronavirus 2 infection: comparison with contemporary and matched pre-COVID-19 controls. J Perinat Med. 2021. https://doi.org/10.1515/JPM-2021-0313.
Flannery DD, Gouma S, Dhudasia MB, Mukhopadhyay S, Pfeifer MR, Woodford EC, et al. Assessment of Maternal and Neonatal Cord Blood SARS-CoV-2 Antibodies and Placental Transfer Ratios. JAMA Pediatr. 2021;175:594–600.
Zimmermann P, Curtis N. Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections. Arch Dis Child. 2020;106:429–39.
Funding
This study was partially funded (BAA 75D301-20-C-08495) by the US Centers for Disease Control and Prevention, which also provided technical assistance related to analysis and interpretation of data and writing the report.
Author information
Authors and Affiliations
Contributions
DDF conceptualized and designed the study, collected data, performed data analysis, and drafted the initial paper. MRP, KB, and DDW collected data and reviewed and revised the paper. MBD, AZB, and TRG collected data, performed data analysis, and reviewed and revised the paper. EEF, SM, SRE, and KRW contributed to the study design and reviewed and revised the paper. MLH and KMP conceptualized and designed the study, supervised data collection and analysis, and reviewed and revised the paper. All authors approved the final paper as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors report there are competing financial interests in relation to the work described. DDF reports receiving grant funding from the Agency for Healthcare Research and Quality (K08HS027468), the Children’s Hospital of Philadelphia, and from two research contracts with the Centers for Disease Control and Prevention. MLH reports research funding from the National Institutes of Health. EEF reports receiving grant funding from the National Institutes of Health (K23HD084727). KMP reports receiving research funding from the National Institutes of Health, from the Children’s Hospital of Philadelphia, and from two contracts with the Centers for Disease Control and Prevention.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Flannery, D.D., Zevallos Barboza, A., Pfeifer, M.R. et al. Perinatal COVID-19 maternal and neonatal outcomes at two academic birth hospitals. J Perinatol 42, 1338–1345 (2022). https://doi.org/10.1038/s41372-022-01446-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-022-01446-x
This article is cited by
-
Neonatal outcomes of maternal prenatal coronavirus infection
Pediatric Research (2023)
-
Management of neonates with maternal prenatal coronavirus infection and influencing factors
Pediatric Research (2023)