Abstract
Background
Advances in technology, data availability, and analytics have helped improve quality of care in the neonatal intensive care unit.
Objective
To provide an in-depth review of artificial intelligence (AI) and machine learning techniques being utilized to predict neonatal outcomes.
Methods
The PRISMA protocol was followed that considered articles from established digital repositories. Included articles were categorized based on predictions of: (a) major neonatal morbidities such as sepsis, bronchopulmonary dysplasia, intraventricular hemorrhage, necrotizing enterocolitis, and retinopathy of prematurity; (b) mortality; and (c) length of stay.
Results
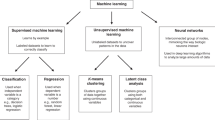
A total of 366 studies were considered; 68 studies were eligible for inclusion in the review. The current set of predictor models are primarily built on supervised learning and mostly used regression models built on retrospective data.
Conclusion
With the availability of EMR data and data-sharing of NICU outcomes across neonatal research networks, machine learning algorithms have shown breakthrough performance in predicting neonatal disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
White RD, Smith JA, Shepley MM. Recommended standards for newborn ICU design, eighth edition. J Perinatol. 2013;33:S2–16.
Ellsworth MA, Lang TR, Pickering BW, Herasevich V. Clinical data needs in the neonatal intensive care unit electronic medical record. BMC Med Inform Decis Mak. 2014;14:92.
De Georgia MA, Kaffashi F, Jacono FJ, Loparo KA. Information technology in critical care: review of monitoring and data acquisition systems for patient care and research. Sci World J. 2015;2015:1–9.
Strickland NH. PACS (picture archiving and communication systems): filmless radiology. Arch Dis Child BMJ Publ Group Ltd. 2000;83:82–6.
Fairchild KD, Aschner JL. HeRO monitoring to reduce mortality in NICU patients. Rrn Dove Press. 2012;2:65–76.
Griffin MP, Lake DE, Bissonette EA, Harrell FE, Shea OTM, Moorman JR. Heart rate characteristics: novel physiomarkers to predict neonatal infection and death. Pediatrics Am Acad Pediatrics. 1999;116:1070.
Fairchild KD, Lake DE. Cross-correlation of heart rate and oxygen saturation in very low birthweight infants: association with apnea and adverse events. Am J Perinatol. 2018;35:463.
Davoudi A, Malhotra KR, Shickel B, Siegel S, Williams S, Ruppert M, et al. Intelligent ICU for autonomous patient monitoring using pervasive sensing and deep learning. Sci Rep. 2019;9:8020. https://doi.org/10.1038/s41598-019-44004-w.
Hee Chung E, Chou J, Brown KA. Neurodevelopmental outcomes of preterm infants: a recent literature review. Transl Pediatr. 2020;9:S3–8.
Internet: The Future of Patient Care Is Now Connected Critical Care at Philips. [cited 2021 Jun 2]. pp. 1–17. Available from: https://www.documents.philips.com/assets/20210121/be28054cfbcf4b358e4cacb701721990.pdf?_gl=1*18u25bv*_ga*NTUxMTA4MzI3LjE2MjQxNjIzNTQ.*_ga_2NMXNNS6LE*MTYyNTIwMjI4Ny40LjEuMTYyNTIwMjQyMy40OA.&_ga=2.92528861.1923540143.1625202287-551108327.1624162354
Internet: CARESCAPE ONE Usability Study. 2020 Jun 24;:1–4. [cited 2021 Jun 2]. Available from: https://www.gehealthcare.com/-/jssmedia/carescape-one-usability-study-wisconsin_jb76705xx_jun24.pdf?rev=-1
Internet: IntelliBridge EC10 Medical device interfacing module. [cited 2021]. Available from: https://www.philips.co.in/healthcare/product/HCNOCTN429/intellibridge-ec10-medical-device-interfacing-module
Internet: BedMasterEx. Real time clinical data acquisition. [cited 2021 Jun 3]. Available from: https://www.anandic.com/en/healthcare-it/bedmasterex/bedmasterex
Internet: Capsule. [cited 2021 Jun 2]. Available from: https://capsuletech.com/critical-care
Singh H, Kaur R, Gangadharan A, IEEE AP, 2018. Neo-bedside monitoring device for integrated neonatal intensive care unit (iNICU). ieeexploreieeeorg. 2018;7:7803–13.
Internet: The iXellence. [cited 2021 Jun 2]. Available from: (www.ixellence.com)
Vincent JL, Suter P, Bihari D, Braining H. Organization of intensive care units in Europe: lessons from the EPIC study. Intensive Care Med. 1997;23:1181–4.
Carayon P, Wetterneck TB, Alyousef B, Brown RL, Cartmill RS, McGuire K, et al. Impact of electronic health record technology on the work and workflow of physicians in the intensive care unit. Int J Med Inform. 2015;84:578–94.
Bodagh N, Archbold RA, Weerackody R, Hawking MKD, Barnes MR, Lee AM, et al. Feasibility of real-time capture of routine clinical data in the electronic health record: a hospital-based, observational service-evaluation study. BMJ Open. 2018;8:e019790.
Internet: Meditech. [cited 2021 Jun 2]. Available from: https://ehr.meditech.com/ehr-solutions/ehr-mobility.
Internet: Allscripts Professional. [cited 2021 Jun 2]. Available from: https://www.allscripts.com/solution/professional/.
Obermeyer Z, Emanuel EJ. Predicting the future - big data, machine learning, and clinical medicine. N. Engl J Med. 2016;375:1216–9.
Mark R. The story of MIMIC. In: Data MC, editor. Secondary Analysis of Electronic Health Records. Cham: Springer International Publishing; 2016. pp. 43–9.
Saeed M, Villarroel M, Reisner AT, Clifford G, Lehman L-W, Moody G, et al. Multiparameter intelligent monitoring in intensive care II (MIMIC-II): a public-access intensive care unit database. Crit Care Med. 2011;39:952.
Johnson AE, Pollard TJ, Shen L, Li-Wei HL, Feng M, Ghassemi M, et al. MIMIC-III, a freely accessible critical care database. Sci Data. 2016;3:1–9.
Internet: The Vermont Oxford Network. [cited 2021 Jun 2]. Available from: https://www.vtoxford.org.
Internet: The CNN Abstractor’s Manual. [cited 2021 Jun 2]. Available from: http://www.canadianneonatalnetwork.org.
Hanson CW 3rd, Marshall BE. Artificial intelligence applications in the intensive care unit. Crit Care Med. 2001;29:427–35.
Kersting K. Machine learning and artificial intelligence: two fellow travelers on the quest for intelligent behavior in machines. Front Big Data. 2018;1:6
Shamout F, Zhu T, Clifton D. Machine learning for clinical outcome prediction. IEEE Rev Biomed Eng. 2021;14:116–26.
Shirwaikar RD, Mago N, U DA, Makkithaya K, K GH. Supervised learning techniques for analysis of neonatal data. 2nd International Conference on Applied and Theoretical Computing and Communication Technology (iCATccT). 2016; pp. 25–31.
Afrin R, Haddad H, Shahriar H. Supervised and unsupervised-based analytics of intensive care unit data. IEEE 43rd annual computer software and applications conference (COMPSAC). 2019; pp. 417–22.
Nemati S, Ghassemi MM, Clifford GD. Optimal medication dosing from suboptimal clinical examples: a deep reinforcement learning approach. Annu Int Conf IEEE Eng Med Biol Soc. 2016;2016:2978–81.
Aniruddh R, Matthieu K, Leo AC, Peter S, Marzyeh G. Continuous state-space models for optimal sepsis treatment: a deep reinforcement learning approach. proceedings of the 2nd machine learning for healthcare conference. PMLR. 2017;68:147–63.
The CRIB (clinical risk index for babies) score: a tool for assessing initial neonatal risk and comparing performance of neonatal intensive care units. The international neonatal network. Lancet.1993;342:193–8.
Lee SK, Aziz K, Dunn M, Clarke M, Kovacs L, Ojah C. et al. Transport risk index of physiologic stability, version II (TRIPS-II): a simple and practical neonatal illness severity score. Am J Perinatol Thieme Med Publishers. 2013;30:395–400.
Gagliardi L, Cavazza A, Brunelli A, Battaglioli M, Merazzi D, Tandoi F, et al. Assessing mortality risk in very low birthweight infants: a comparison of CRIB, CRIB-II, and SNAPPE-II. Arch Dis Child Fetal Neonatal Ed. 2004;89:F419–22.
Parry G, Tucker J, Tarnow-Mordi W, Group UNSSC. CRIB II: an update of the clinical risk index for babies score. Lancet. 2003;361:1789–91.
Kim SY, Kim S, Cho J, Kim YS, Sol IS, Sung Y, et al. A deep learning model for real-time mortality prediction in critically ill children. Crit Care. 2019;23:279.
Suresh H, Hunt N, Johnson A, Celi LA, Szolovits P, Ghassemi M. Clinical intervention prediction and understanding with deep neural networks. Doshi-Velez F, Fackler J, Kale D, Ranganath R, Wallace B, Wiens J, editors. Proceedings of Machine Learning Research. Proceedings of Machine Learning Research: PMLR; 2017;68:322–37.
McGregor C. Big data in neonatal intensive care. Computer. IEEE. 2013;46:54–9.
Addy DP. “Neonatal” Is the first 28 days of life. Pediatrics. 1975;55:571.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Thébaud B, Goss KN, Laughon M, Whitsett JA, Abman SH, Steinhorn RH. et al. Bronchopulmonary dysplasia. Nat Rev Dis Prim. 2019;5:78.
Özek E, Kersin SG. Intraventricular hemorrhage in preterm babies. Turk Pediatr Ars Kare Publ. 2020;55:215–21.
Neu J. Necrotizing enterocolitis: the future. Neonatology. 2020;117:240–4.
Kim SJ, Port AD, Swan R, Campbell JP, Chan RVP, Chiang MF. Retinopathy of prematurity: a review of risk factors and their clinical significance. Surv Ophthalmol. 2018;63:618–37.
Feldman K, Chawla NV. Admission duration model for infant treatment (ADMIT). IEEE; 2014. pp. 583–7.
Zernikow B, Holtmannspötter K, Michel E, Hornschuh F, Groote K, Hennecke KH. Predicting length of stay in preterm neonates 52. Pediatric Res. 1997;42:393–3.
Bender GJ, Koestler D, Ombao H, McCourt M, Alskinis B, Rubin LP, et al. Neonatal intensive care unit: predictive models for length of stay. J Perinatol. 2012;33:147–53.
Thompson B, Elish K, Steele R. Machine learning-based prediction of prolonged length of stay in newborns. 2018 17th IEEE International Conference on Machine Learning and Applications (ICMLA). IEEE;2018,1454–9.
Lee HC, Bennett MV, Schulman J, Gould JB. Accounting for variation in length of NICU stay for extremely low birth weight infants. Nat Publ Group. 2013;33:872–6.
Lee HC, Bennett MV, Schulman J, Gould JB, Profit J. Estimating length of stay by patient type in the neonatal intensive care unit. Am J Perinatol. 2016;33:751–7.
Singh H, Cho SJ, Gupta S, Kaur R, Sunidhi S, Saluja S, et al. Designing a bed-side system for predicting length of stay in a neonatal intensive care unit. Sci Rep. 2021:1–13.
Geoghegan L, Scarborough A, Wormald JC, Harrison CJ, Collins D, Gardiner M. et al. Automated conversational agents for post-intervention follow-up: a systematic review. BJS Open. 2021;5:zrab070.
Kalaniti K, Mugarab-Samedi V, Riehl A, Bingham W, Daspal S. Web-based Camera (NICView) as a sense of proximity tool: a Quality-Improvement initiative for parents of neonates admitted in the NICU. Paediatrics Child Health. 2020;25:e12–3.
Singh H, Mallaiah R, Yadav G, Verma N, Sawhney A, Brahmachari SK. iCHRCloud: web & mobile based child health imprints for smart healthcare. J Med Sys. 2018;42:1–12.
Singh H, Kusuda S, McAdams RM, Gupta S, Kalra J, Kaur R, et al. Machine learning-based automatic classification of video recorded neonatal manipulations and associated physiological parameters: a feasibility study. Child Multidiscip Digital Publ Inst. 2021;8:1.
Internet: Vidyo. [cited 2021 Jun 4. Available from: https://www.vidyohealth.com/.
Moorman JR, Carlo WA, Kattwinkel J, Schelonka RL, Porcelli PJ, Navarrete CT, et al. Mortality reduction by heart rate characteristic monitoring in very low birth weight neonates: a randomized trial. J Pediatrics. 2011;159:900–1.
Moorman JR, Delos JB, Flower AA, Cao H, Kovatchev BP, Richman JS. et al. Cardiovascular oscillations at the bedside: early diagnosis of neonatal sepsis using heart rate characteristics monitoring. Physiol Measurement. 2011;32:1821–32.
Saria S, Rajani AK, Gould J, Koller D, Penn AA. Integration of early physiological responses predicts later illness severity in preterm infants. Science translational medicine. Am Assoc Advancement Sci. 2010;2:48ra65–5.
Mahieu LM, De Muynck AO, De Dooy JJ, Laroche SM, Van Acker KJ. Prediction of nosocomial sepsis in neonates by means of a computer-weighted bedside scoring system (NOSEP score). Critical Care Med. 2000;28:2026–33.
Helguera-Repetto AC, Soto-Ramírez MD, Villavicencio-Carrisoza O, Yong-Mendoza S, Yong-Mendoza A, León-Juárez M, et al. Neonatal sepsis diagnosis decision-making based on artificial neural networks. Front Pediatr Front. 2020;8:1–10.
Laughon MM, Langer JC, Bose CL, Smith PB, Ambalavanan N, Kennedy KA, et al. Prediction of bronchopulmonary dysplasia by postnatal age in extremely premature infants. Am J Respir Crit Care Med. 2011;183:1715–22.
Cuna A, Liu C, Govindarajan S, Queen M, Dai H, Truog WE. Usefulness of an online risk estimator for bronchopulmonary dysplasia in predicting corticosteroid treatment in infants born preterm. J Pediatr. 2018;197:23–8.
May C, Patel S, Kennedy C, Pollina E, Rafferty GF, Peacock JL, et al. Prediction of bronchopulmonary dysplasia. Arch Dis Child Fetal Neonatal Ed. 2011;96:F410–6.
Buzkova K, Suchomel J. Use of electrical impedance tomography for quantitative evaluation of disability level of bronchopulmonary dysplasia. IEEE; 2013, pp. 1–4.
Jensen EA, DeMauro SB, Kornhauser M, Aghai ZH, Greenspan JS, Dysart KC. Effects of multiple ventilation courses and duration of mechanical ventilation on respiratory outcomes in extremely low-birth-weight infants. JAMA Pediatrics Am Med Assoc. 2015;169:1011–7.
Tsuji M, Saul JP, Plessis du A, Eichenwald E, Sobh J, Crocker R, et al. Cerebral intravascular oxygenation correlates with mean arterial pressure in critically ill premature infants. Pediatrics Am Acad Pediatrics. 2000;106:625–32.
Sullivan BA, McClure C, Hicks J, Lake DE, Moorman JR, Fairchild KD. Early heart rate characteristics predict death and morbidities in preterm infants. J Pediatrics Elsevier. 2016;174:57–62.
Vergales BD, Zanelli SA, Matsumoto JA, Goodkin HP, Lake DE, Moorman JR, et al. Depressed heart rate variability is associated with abnormal EEG, MRI, and death in neonates with hypoxic ischemic encephalopathy. Am J Perinatol Thieme Med Publ. 2013;31:855–62.
Sortica da Costa C, Placek MM, Czosnyka M, Cabella B, Kasprowicz M, Austin T, et al. Complexity of brain signals is associated with outcome in preterm infants. J Cereb Blood Flow Metab. 2017;37:3368–79.
Galderisi A, Zammataro L, Losiouk E, Lanzola G, Kraemer K, Trevisanuto D. et al. Continuous glucose monitoring linked to an artificial intelligence risk index: early footprints of intraventricular hemorrhage in preterm neonates. Diabetes Technol Ther. 2019;21:146–53.
Tam EW, Haeusslein LA, Bonifacio SL, Glass HC, Rogers EE, Jeremy RJ, et al. Hypoglycemia is associated with increased risk for brain injury and adverse neurodevelopmental outcome in neonates at risk for encephalopathy. J Pediatr. 2012;161:88–93.
Schmid MB, Reister F, Mayer B, Hopfner RJ, Fuchs H, Hummler HD. Prospective risk factor monitoring reduces intracranial hemorrhage rates in preterm infants. Dtsch Arztebl Int. 2013;110:489.
Doheny KK, Palmer C, Browning KN, Jairath P, Liao D, He F, et al. Diminished vagal tone is a predictive biomarker of necrotizing enterocolitis‐risk in preterm infants. Neurogastroenterol Motil. 2014;26:832–40.
Stone ML, Tatum PM, Weitkamp JH, Mukherjee AB, Attridge J, McGahren ED. et al. Abnormal heart rate characteristics before clinical diagnosis of necrotizing enterocolitis. J Perinatol. 2013;33:847–50.
Ibáñez V, Couselo M, Marijuán V, Vila JJ, García-Sala C. Could clinical scores guide the surgical treatment of necrotizing enterocolitis?. Pediatr Surg Int. 2012;28:271–6.
Gephart SM, Spitzer AR, Effken JA, Dodd E, Halpern M, McGrath JM. Discrimination of GutCheck NEC: a clinical risk index for necrotizing enterocolitis. J Perinatol. 2014;34:468–75.
Hooven T, Lin YC, Salleb-Aouissi A. Multiple instance learning for predicting necrotizing enterocolitis in premature infants using microbiome data. In Proceedings of the ACM Conference on Health, Inference, and Learning 2020 (pp. 99–109).
Irles C, González-Pérez G, Carrera Muiños S, Michel Macias C, Sánchez Gómez C, Martínez-Zepeda A, et al. Estimation of neonatal intestinal perforation associated with necrotizing enterocolitis by machine learning reveals new key factors. Int J Environ Res Public Health. 2018;15:2509.
Wu C, Löfqvist C, Smith LEH, VanderVeen DK, Hellström A. WINROP Consortium FT. Importance of early postnatal weight gain for normal retinal angiogenesis in very preterm infants: a multicenter study analyzing weight velocity deviations for the prediction of retinopathy of prematurity. Arch Ophthalmol. 2012;130:992–9.
Kaempf JW, Kaempf AJ, Wu Y, Stawarz M, Niemeyer J, Grunkemeier G. Hyperglycemia, insulin and slower growth velocity may increase the risk of retinopathy of prematurity. J Perinatol. 2019;31:1–7.
Eckert GU, Filho JBF, Maia M, Procianoy RS. A predictive score for retinopathy of prematurity in very low birth weight preterm infants. Eye. 2019;26:1–7.
Binenbaum G, Ying G-S, Tomlinson LA. Group FTPGAROPG-RS. Validation of the children’s hospital of philadelphia retinopathy of prematurity (CHOP ROP) model. JAMA Ophthalmol. 2017;135:871–7.
Freitas AM, Mörschbächer R, Thorell MR, Rhoden EL. Incidence and risk factors for retinopathy of prematurity: a retrospective cohort study. Int J Retina Vitreous. 2018;4:1–8.
Sullivan BA, Wallman-Stokes A, Isler J, Sahni R, Moorman JR, Fairchild KD, et al. Early pulse oximetry data improves prediction of death and adverse outcomes in a two-center cohort of very low birth weight infants. Am J Perinatol Thieme Med Publishers. 2018;35:1331–8.
Brown JM, Campbell JP, Beers A, Chang K, Ostmo S, Chan RP, et al. Automated diagnosis of plus disease in retinopathy of prematurity using deep convolutional neural networks. JAMA Ophthalmol. 2018;136:803–10.
Owen LA, Morrison MA, Hoffman RO, Yoder BA, DeAngelis MM. Retinopathy of prematurity: A comprehensive risk analysis for prevention and prediction of disease. PloS One. 2017;12:e0171467.
Richardson DK, Gray JE, McCormick MC, Workman K, Goldmann DA. Score for neonatal acute physiology: a physiologic severity index for neonatal intensive care. Pediatrics. 1993;91:617.
Richardson DK, Phibbs CS, Gray JE, McCormick MC, Workman-Daniels K, Goldmann DA. Birth weight and illness severity: independent predictors of neonatal mortality. Pediatrics Am Acad Pediatrics. 1993;91:969–75.
Wisnuwardani DN, Arif Sampurna MT, Utomo MT, Etika R. Neonatal therapeutic intervention scoring system (NTISS) in rural country: mortality and length of stay (LOS) predictive score in preterm infant. Ind J Forensic Med Toxicol. 2020;14:862–7.
Lee SK, Zupancic JAF, Pendray M, Thiessen P, Schmidt B, Whyte R, et al. Transport risk index of physiologic stability: a practical system for assessing infant transport care. J Pediatrics. 2001;139:220–6.
Parry G, Tucker J, Tarnow-Mordi W, Group UNSSC. CRIB II: an update of the clinical risk index for babies score. Lancet. 2003;361:1789–91.
Richardson DK, Corcoran JD, Escobar GJ, Lee SK. SNAP-II and SNAPPE-II: simplified newborn illness severity and mortality risk scores. J Pediatr. 2001;138:92–100.
Skarsgard ED, MacNab YC, Qiu Z, Little R, Lee SK. SNAP-II predicts mortality among infants with congenital diaphragmatic hernia. J Perinatol Nat Publ Group. 2005;25:315–9.
Muktan D, Singh RR, Bhatta NK, Shah D. Neonatal mortality risk assessment using SNAPPE-II score in a neonatal intensive care unit. BMC Pediatri. 2019;19:1–4.
Beltempo M, Shah PS, Ye XY, Afifi J, Lee S, Mcmillan DD, et al. SNAP-II for prediction of mortality and morbidity in extremely preterm infants. J Maternal Fetal Neonatal Med. 2019;32:2694–701.
Lee SK, Mcmillan DD, Ohlsson A, Pendray M, Synnes A, Whyte R, et al. Variations in practice and outcomes in the Canadian NICU network: 1996–1997. Pediatrics. 2000;106:1070.
Aliaga S, Boggess K, Ivester TS, Price WA. Influence of neonatal practice variation on outcomes of late preterm birth. Am J Perinatol. 2014;31:659–66.
Baskaran V, Bajan I, Shah B, Prescod FI, James A. A knowledge management based approach for mortality prediction in the neonatal intensive care unit. In 2011 Developments in E-systems Engineering 2011(pp. 122–5). IEEE.
Kong X, Xu F, Wu R, Wu H, Ju R, Zhao X, et al. Neonatal mortality and morbidity among infants between 24 to 31 complete weeks: a multicenter survey in China from 2013 to 2014. BMC Pediatr. 2016;26:1–8.
Podda M, Bacciu D, Micheli A, Bellù R, Placidi G, Gagliardi L. A machine learning approach to estimating preterm infants survival: development of the preterm infants survival assessment (PISA) predictor. Sci Rep. 2018;8:13743.
Shi P, Gangopadhyay A, Owens C, Blunt B, Grogan C A hybrid model using LSTM and decision tree for mortality prediction and its application in provider performance evaluation. IEEE; 2019. pp. 2773–81.
Rinta-Koski O-P, Särkkä S, Hollmén J, Leskinen M, Andersson S. Gaussian process classification for prediction of in-hospital mortality among preterm infants. Neurocomputing. 2018;298:134–41.
Jaskari J, Myllärinen J, Leskinen M, Rad AB, Hollmén J, Andersson S, et al. Machine learning methods for neonatal mortality and morbidity classification. IEEE Access. 2020;8:123347–58.
Meister AL, Doheny KK, Travagli RA. Necrotizing enterocolitis: It’s not all in the gut. Exp Biol Med. 2020;245:85–95. PMID: 31810384; PMCID: PMC7016421.
Kliegman RM, Walsh MC. Neonatal necrotizing enterocoli- tis: pathogenesis, classification, and spectrum of illness. Curr Probl Pediatr. 1987;17:213–88.
Hu Y, Lee VCS, Tan K. An application of convolutional neural networks for the early detection of late-onset neonatal sepsis. 2019 International Joint Conference on Neural Networks (IJCNN). IEEE; 2019 1–8.
Fairchild KD, Nagraj VP, Sullivan BA, Moorman JR, Lake DE. Oxygen desaturations in the early neonatal period predict development of bronchopulmonary dysplasia. Pediatr Res. 2019;85:987–93.
Shi Y, Payeur P, Frize M, Bariciak E. Thermal and RGB-D imaging for necrotizing enterocolitis detection. IEEE; 2020. pp. 1–6.
Sun Y, Kaur R, Gupta S, Paul R, Das R, Cho SJ, et al. Development and validation of high definition phenotype-based mortality prediction in critical care units. JAMIA Open. 2021;4:1–13.
Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187:1–7.
Hwang M, Tierradentro-García LO, Dennis RA, Anupindi SA. The role of ultrasound in necrotizing enterocolitis. Pediatr Radiol. 2021 https://doi.org/10.1007/s00247-021-05187-5. Epub ahead of print. PMID: 34654968.
Shi Y, Payeur P, Frize M, Bariciak E. “Thermal and RGB-D Imaging for Necrotizing Enterocolitis Detection,” 2020 IEEE International Symposium on Medical Measurements and Applications (MeMeA), 2020, pp. 1–6, https://doi.org/10.1109/MeMeA49120.2020.9137344.
Author information
Authors and Affiliations
Contributions
RM, HS, RK, HB conceptualized and designed the study, analysis, or interpretation of data was done by HB, HS, RK. Drafting of the manuscript was done by RM, HS, and RK. Critical revision of the manuscript for important intellectual content was done by all authors. Statistical analysis was done by HS, HB. Funding was Obtained HS. Administrative, technical, or material support was provided by HS, RK, RM. The project was supervised by RM.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
McAdams, R.M., Kaur, R., Sun, Y. et al. Predicting clinical outcomes using artificial intelligence and machine learning in neonatal intensive care units: a systematic review. J Perinatol 42, 1561–1575 (2022). https://doi.org/10.1038/s41372-022-01392-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-022-01392-8
This article is cited by
-
Artificial intelligence in the neonatal intensive care unit: the time is now
Journal of Perinatology (2024)
-
Predicting the effectiveness of drugs used for treating cardiovascular conditions in newborn infants
Pediatric Research (2024)
-
The past, current, and future of neonatal intensive care units with artificial intelligence: a systematic review
npj Digital Medicine (2023)
-
PD(AI): the role of artificial intelligence in the management of patent ductus arteriosus
Journal of Perinatology (2023)
-
An efficient random forest algorithm-based telemonitoring framework to predict mortality and length of stay of patients in ICU
Multimedia Tools and Applications (2023)