Abstract
Aim
The aim of this study is to determine the incidence of metabolic bone disease (MBD) and assess the risk factors for development radiologic evidence of MBD.
Methods
Preterm infants with gestational age ≤32 weeks and birth weight ≤1500 g were included in this prospective study. Metabolic bone disease was defined as hypophosphatemia (phosphorus levels <4 mg/dl), ALP levels >450 U/L, or radiologic findings of MBD at four weeks of age.
Results
The study included 254 infants (gestational age: 29 (27–30) weeks, birth weight: 1130 g (960–1300)). Metabolic bone disease was diagnosed in 96 patients (37%); 48 infants had only radiologic evidence of MBD, 24 infants had only biochemical diagnosis of MBD, and 24 infants had both radiologic evidence of MBD and biochemical diagnosis of MBD.
Conclusions
Our results showed that radiologic evidence of MBD existed in some infants with normal biochemical results. That finding may guide further development of screening programs for MBD.
Similar content being viewed by others
Introduction
Osteopenia of prematurity, also called metabolic bone disease (MBD) or rickets of prematurity, describes the reduction in bone mineral content of the preterm infant for gestational age with biochemical and radiologic features. It is primarily result of inadequate calcium and phosphorus intake to meet bone growth demands. The incidence of MBD is estimated to be 23–32% in infants with a birth weight <1500 g and 50% in infants weighting <1000 g [1, 2]. It is known that the main risk factor is reduced bone mineralization following premature delivery, so low gestational age and birth weight are main risk factors [1,2,3,4]. Besides, many other risk factors were identified; prolonged total parenteral nutrition (TPN), feeding with unfortified human milk, use of medications like diuretics, steroids, and methylxanthines, as well as neonatal conditions such as sepsis, cerebral pathology, muscular disorders, and sedation during mechanical ventilation may result in prolonged periods of immobility, as a risk for calcium loss and demineralization [1, 4].
MBD of prematurity typically manifests between 3 and 12 weeks of age [2, 4]. Laboratory findings that are suggestive of MBD include a low serum phosphorus level <3.5–4 mg/dl and increased alkaline phosphatase activity of greater than 1000 IU/ml [5,6,7,8]. Premature infants are advised to be evaluated with wrist radiography [2,3,4]. Nevertheless, diagnosis of MBD depends not solely on laboratory studies but presence of radiographic findings. Currently, no single value of alkaline phosphatase or phosphate levels is related to radiological findings of MBD [7]. Although it was demonstrated that there existed huge variations in the method of screening for MBD of prematurity among individual neonatal intensive care units (NICU) [9], The American Academy of Pediatrics Committee on Nutrition (AAP-CON) recommends screening all very low birth weight (VLBW) infants for MBD with serum phosphate or alkaline phosphates (ALP) starting at four to five weeks of age [10].
In this study, we aimed to determine the incidence of MBD and assess the risk factors for development radiologic evidence of MBD.
Materials and methods
A prospective cohort study was conducted at Etlik Zübeyde Hanım Women’s Health Teaching and Research Hospital, Ankara, Turkey between January 2018 and June 2019. Preterm infants who survived until four weeks of age with GA ≤32 weeks and BW ≤1500 g were included in the study. Infants with major congenital anomalies or those who died or transferred out of NICU before the date of evaluation, four weeks of age, were excluded from the study. Written informed consent was obtained from guardians or parents of children. Institutional ethics committee was approved the study.
Included infants were screened for MBD of prematurity by measuring serum of ALP levels, serum calcium, and serum phosphate levels and left wrist x-ray at fourth week of age. MBD was defined as hypophosphatemia (phosphorus levels <4 mg/dl), ALP levels >450 U/L, or radiologic findings of MBD at four weeks of age.
Wrist x-rays were evaluated by a radiologist masked to the biochemical results and the clinical data of the infants. Radiologic evidence of MBD was defined according to screening criteria of Koo et al.; presence of loss of dense zone of provisional calcification at metaphysis, increased submetaphyseal lucency, thinning of cortex, fraying, splaying, or cupping of metaphysis [11]. After the radiologic evaluation, infants with and without radiologic evidence of MBD were grouped.
The documented antenatal and clinical variables were gestational age, birth weight, maternal age, multiple gestation, antenatal steroid administration (two doses of 12 mg betamethasone given intramuscularly before delivery), maternal preeclampsia, mode of delivery, gender, 5-min Apgar score, being small for gestational age, sepsis (early or late; proven or probable), caffeine citrate treatment, duration of parenteral nutrition, time to regain birth weight, mechanical ventilation and noninvasive ventilation treatment, ventilation treatment more than 14 days (mechanical and noninvasive ventilation), oxygen requirement at 28th days of life, chronic lung disease (oxygen requirement at 36th postmenstrual age), respiratory distress syndrome requiring surfactant treatment, patent ductus arteriosus (medically treated), necrotizing enterocolitis (modified Bell’s staging criteria ≥grade 2), severe retinopathy of prematurity (≥stage 2) were analyzed. The data from infants with and without radiologic evidence of MBD were compared.
Parenteral and enteral nutrition policy of our NICU
All infants received TPN in the 1st day of life [amino acid solution (2 g/kg; Primene-10%; Baxter, S.A. Belgium), lipid solution (1 g/kg; ClinOleic-20%; Baxter, S.A. Belgium), glucose]. The amino acid/lipid doses were increased to 4/3 g/kg on the 3rd day. Enteral feeding was started as 10 ml/kg with breast milk within 2 days and after 48 h if preterm formula was the initial nutrition. Trace elements and water–lipid soluble vitamins were supplemented to TPN after the 1st week of life. During parenteral nutrition calcium (Calcium Gluconate), phosphate (Sodium glycerophosphate) were added as fallows; days 1–3: calcium; 27 mg/kg, day 4 and later; calcium 47 mg/kg and phosphate 31 mg/kg). Glycophos® contains sodium glycerophosphate which, 2 meq sodium and 1 mmol phosphate for 1 ml.
Feedings were advanced 20–30 ml/kg/day. TPN volume was decreased proportionally and continued until 75% of total volume was obtained from enteral feeding. In total, 1.1 g of Eoprotin® (Aptamil® Milupa breast milk fortifier) was added to every 25 ml human milk when enteral intake is greater than 100 ml/kg per day and 400 IU vitamin D was given after the infants achieved full enteral feeding. In case of inadequate human milk, infants fed with preterm formula (Prematil®-Milupa).
All infants were fed with either fortified human milk (calcium content 92 mg/dl and phosphorus 52 mg/dl) or preterm formulas (calcium 120 mg/dl and phosphorus 60 mg/dl).
Statistical analyses were conducted using the SPSS V15.0 (SPSS Inc., Chicago, IL). Categorical variables were presented as n/N (%), and were compared by Chi-square test, Fisher exact test, or Yates correction, where appropriate. The distribution of numerical variables was investigated and compared between two groups by Mann–Whitney U test or independent samples t-test, where appropriate. The numeric variables were presented as means with respective standard deviation, and as medians with their interquartile range (IQR) where appropriate. Statistical significance was accepted if p value ≤0.05. Infants with and without radiologic evidence of MBD were compared for probable risk factors that infants were exposed before radiologic evaluation. The multivariate analysis of logistic regression was performed with variables of p < 0.1 in the univariate analysis. Hosmer–Lemeshow goodness of fit statistics were used to assess model fit. Odds ratios and 95% confidence intervals for each risk factor were determined. Positive predictive value, sensitivity and specificity for biochemical tests to diagnose radiologic evidence of MBD were calculated.
Results
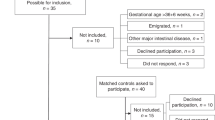
During study period, 318 eligible VLBW infants were admitted. Of these, 254 infants were included, and 64 infants were excluded; multiple congenital anomalies (n = 14), no parental consent (n = 16), died (n = 30), and transferred out of NICU (n = 4).
Median birth weight was 1130 (960–1300) g and gestational age of the study population was 29 (27–30) weeks. The ratio of extremely low birth weight (ELBW) preterm infants was found to be 28.7%. None of the infants were given steroid regimen or diuretic treatment for probable chronic lung disease in the 1st month of life. MBD was diagnosed in 96 patients (37%); 48 infants had only radiologic evidence of MBD, 24 infants had only biochemical diagnosis of MBD, and 24 infants had both radiologic evidence of MBD and biochemical diagnosis of MBD.
Clinical characteristics of included infants and evaluation of clinical characteristics of 72 infants with radiologic evidence of MBD and 192 infants without radiologic evidence of MBD were detailed in Table 1.
Infants with radiologic evidence of MBD were found to have significantly lower birth weight, gestational age, have higher incidence of sepsis and medically treated patent ductus arteriosus, receive longer duration of parenteral nutrition and duration of ventilation. It was found that incidence of ventilation treatment more than 14 days and requirement of oxygen at 28th postnatal day were higher in infants with radiologic evidence of MBD compared to infants without radiologic evidence of MBD.
Gestational age, preeclampsia, sepsis, patent ductus arteriosus, duration of parenteral nutrition, ventilation requirement more than 14 days, oxygen dependency at 28th day were put in the regression model. We found that ventilation requirement more than 14 days was independent risk factor for development of radiologic evidence of MBD as shown in Table 2.
The mean level of serum ALP was significantly higher (IU/L; 577 ± 281 vs 412 ± 168, p < 0.001) and serum phosphate level was significantly lower (mg/dl; 4.2 ± 1.1 vs 5.1 ± 1.0, p < 0.001) in infants with radiologic evidence of MBD compared to infants without radiologic evidence of MBD, whereas serum calcium level was found to be similar and in normal range in both groups (Table 3).
The accuracy of biochemical tests (serum ALP, calcium, and phosphate levels) to detect radiologic finding was found to be—sensitivity: 33%, specificity: 87%, PPV: 50%, and NPV: 76%. The 48 infants who had only radiologic evidence of MBD had median serum phosphorus level of 5 mg/dl (IQR: 4.0–5.7 mg/dl) and median serum ALP of 410 IU/l (IQR: 324–462 IU/l).
Discussion
The MBD of prematurity is characterized by demineralization of bone arising from inadequate provision of calcium and phosphorus, both prenatally and postnatally [1]. It is difficult to quantify the exact incidence of MBD of prematurity, due to differences in terminology and diagnostic criteria. However, the current incidence of MBD may be decreasing owing to improvements in care of premature infants. Nowadays MBD is more commonly seen in ELBW infants. We showed that the incidence of MBD in VLBW infants was 37.7%, and radiologic evidence of MBD was present in 28% of the infants. The incidence of radiologic evidence of MBD in ELBW infants was found to be 50% in our study, where 30.9% in study of Viswanathan et al. [12]. There are also important cohort studies evaluating MBD of prematurity from Turkey. The incidence of osteopenia was reported as 14.9% among babies <32 weeks GA (81/462) [13]. In another single center study from Turkey osteopenia was present in 26.6% of babies ≤34 weeks GA (33/126) [14]. The diagnosis was based on evaluation of serum ALP and phosphorus levels in those studies.
There is no universal consensus on definition of MBD. Given the high risk of MBD in preterm infants VLBW infants should be evaluated throughout the hospitalization for early detection. Preterm infants are screened for MBD by measuring serum calcium, phosphorus, and ALP at four weeks of age according to AAP-CON recommendations in our unit. As to AAP, serum phosphorus level < 4 mg/dl and serum ALP level >500 IU is suggestive of MBD, however, it is stated that ALP has conflicting evidence about its sensitivity and specificity [10]. Thus, the cut-off values that were used for diagnosis of MBD in our study is acceptable. We also obtained radiographs of the wrist to evaluate the radiologic evidence of MBD.
MBD of prematurity typically manifests between 3 and 12 weeks of age and clinically apparent in minority of cases [1, 2, 4]. Demirel et al. reported early MBD in 26.6% and late MBD in 41.9% among preterm infants with GA ≤34 weeks [14]. We screened VLBW infants for MBD at four weeks of age, as recommended by AAP-CON [10]. Among the included infants, there were 48 infants with high ALP and low P levels, which 24 of them had normal x-ray. In particular, 48 infants had radiologic evidence of MBD with normal biochemical results. We think that, this result is really important to create the awareness about possibility of early radiologic evidence in the absence of biochemical diagnosis of MBD.
In this study, the accuracy of biochemical tests to detect radiologic finding was found to be—sensitivity: 33%, specificity: 87%, PPV: 50%, and NPV: 76%. Two-thirds of the infants with osteopenia did not get the diagnosis of MBD with regards to their biochemical test results. Those infants had serum phosphorus (median IQR: 5 (4.0–5.7) and ALP (median IQR: 410 (324–462)). These results should remind the clinicians the possibility of radiologic evidence of rickets in the preterm infants with normal biochemical tests. Viswanathan et al. found that ALP peaked at 2nd month in ELBW infants [12]. Thus, our findings may point to importance of early evaluation of x-ray may be more appropriate with regards to low PPV and sensitivity of tests to define radiologic evidence of MBD. This can allow clinicians to perform early interventions including feeding and physical exercise protocols.
Clinical manifestations of MBD vary from abnormal biochemistry to overt rickets (poor weight gain, growth failure, frontal bossing, swelling of costochondral joints of ribs, and swelling of ankles and wrist joints). However, preterm infants with MBD usually represent few symptoms until further disease process [1, 2]. Biochemical abnormalities as low serum phosphorus and elevated serum ALP levels, detected on regular neonatal screening are initial suggestions [1, 5]. Similarly in this study, preterm infants with MBD demonstrated no symptoms when it was detected. In severe cases, MBD may present as fractures. Pain on handling, swelling, tenderness, and deformity at fracture site may be noted or may be detected incidentally on radiographs [15]. None of our patients had fracture on follow up.
Severe osteopenia may also lead to respiratory difficulties and failure to wean off ventilator support due to excessive chest wall compliance [1, 3]. On the other hand, chronic lung disease was said to be a risk factor for developing MBD of prematurity [16]. However, in this study chronic lung disease was found to develop more in infants with MBD (11.1%) than infants without MBD (3.3%), p = 0.014. Further studies are needed to investigate the effect of MBD and chronic lung disease on development of each of them.
Prematurity, prolonged parenteral nutrition, feeding with unfortified human milk, chronic illness, and the use of hypercalciuric medications, and methylxanthines are known risk factors for development of MBD of prematurity [3]. Although in this study, preterm infants with MBD were found to be more premature, and have longer parenteral nutrition and more sepsis diagnosis compared to infants without MBD, none of those variables was found to be an independent risk factor for development of MBD. Remarkably, it was demonstrated that longer duration of mechanical ventilation is an independent risk factor for development of MBD in our cohort. The Cochrane reviewed the effect of physical exercise on bone development and growth of premature infants and stated that current data in not enough to suggest standardized physical activity programs for premature infants to improve bone health [17]. Yet, a recent review evaluated 14 studies investigated effect of exercise on bone mineralization and emphasized that passive range of motion exercise of the extremities may lead to increased bone mineralization [18]. Thus, long-term mechanical ventilation requirement as an independent predictor of MBD in this study might be explained as to the decrease of physical activity due to both medical condition of the infant and less handling by NICU staff.
Our prospective study showed that VLBW infants may have osteopenia findings as early as fourth week of life in the absence of abnormal biochemical tests. Large sample size, strict enteral, and parenteral feeding protocol of our unit are the strengths of our study. We are aware that MBD is a developmental condition and our study has some limitations. In particular, we do not record either the biochemical results or the x-ray findings in the follow up period. So sequential values of phosphorus and/or ALP during the hospitalization lack in this study. Additionally, we did not evaluate serum PTH and vitamin D levels to avoid risk of anemia due to phlebotomy. Moreover, the exact volume of human milk or formula feeding was not calculated. As the long-term-follow up of NICU graduates’ is out of the scope of our study, out-patient clinical data of the infants were not recorded. Besides we are aware that MBD is now more commonly seen in ELBW infants and the patient population we included was relatively greater than the infants at the highest risk for MBD development. As, MBD screening is recommended in all VLBW infants, we prefer to conduct our study in VLBW population. Nevertheless, in this study we would be able to demonstrate that longer duration of mechanical ventilation is an independent risk factor for development of MBD in VLBW infants.
It is well established that MBD is a complication of preterm delivery with no universal consensus regarding its definition and screening. Screening at fourth week of postnatal life showed that radiologic evidence of MBD existed in some infants with normal biochemical results. That finding may guide further development of screening programs for MBD. Additionally, physical exercise programs to improve bone health of NICU infants must be subject of further trials.
References
Rigo J, Mohamed MW, Curtis M. Disorders of calcium, phosphorus, and magnesium metabolism. In: Martin RJ, Fanaroff AA, Walsh MC, editors. Fanaroff and Martin’s neonatal-perinatal medicine diseases of the fetus and infant. 9th ed. St. Louis: Elsevier; 2011. p. 1523–56.
Sharp M. Bone disease of prematurity. Early Hum Dev. 2007;83:653–8.
Harrison CM, Johnson K, McKechnie E. Osteopenia of prematurity: a national survey and review of practice. Acta Paediatr. 2008;97:407–13.
Vachharajani AJ, Mathur AM, Rao R. Metabolic bone disease of prematurity. Neoreviews. 2009;10:e402–11.
Mitchell SM, Rogers SP, Hicks PD, Hawthorne KM, Parker BR, Abrams SA. High frequencies of elevated alkaline phosphatase activity and rickets exist in extremely low birth weight infants despite current nutritional support. BMC Pediatr. 2009;9:47.
Faerk J, Peitersen B, Petersen S, Michaelsen KF. Bone mineralisation in premature infants cannot be predicted from serum alkaline phosphatase or serum phosphate. Arch Dis Child Fetal Neonatal Ed. 2002;87:F133–6.
Hung YL, Chen PC, Jeng SF, Hsieh CJ, Peng SF, Yen RF, et al. Serial measurements of serum alkaline phosphatase for early prediction of osteopaenia in preterm infants. J Paediatr Child Health. 2011;47:134–9.
Pieltain C, Rigo J. Early mineral metabolism in very-low-birth-weight infants. J Pediatr Gastroenterol Nutr. 2014;58:393.
Kelly A, Kovatch KJ, Garber SJ. Metabolic bone disease screening practices among U.S. neonatologists. Clin Pediatrics. 2014;53:1077–83.
Abrams SA, The Committee on Nutrition. Calcium and vitamin D requirements of enterally fed preterm infants. Pediatrics. 2013;131:1676–83.
Koo WW, Gupta JM, Nayanar VV, Wilkinson M, Posen S. Skeletal changes in preterm infants. Arch Dis Child. 1982;57:447–52.
Viswanathan S, Khasawneh W, McNelis K, Dykstra C, Amstadt R, Super DM, et al. Metabolic bone disease: a continued challenge in extremely low birth weight infants. J Parenter Enter Nutr. 2014;38:982–90.
Cakır U, Tayman C. The effect of thyroid functions on osteopenia of prematurity in preterm infants. J Pediatr Endocrinol Metab. 2019;32:65–70.
Demirel U, Özek E, Bereket A, Demirel B, Topuzoğlu A, Akman İ. Does transient hypothyroxinemia influence metabolic bone disease of prematurity? J Matern Fetal Neonatal Med. 2013;26:1844–9.
Dabezies EJ, Warren PD. Fractures in very low birth weight infants with rickets. Clin Orthop Relat Res. 1997;335:233–9.
Lee SM, Namgung R, Park MS, Eun HS, Park KI, Lee C. High incidence of rickets in extremely low birth weight infants with severe parenteral nutrition-associated cholestasis and bronchopulmonary dysplasia. J Korean Med Sci. 2012;27:1552–5.
Schulzke SM, Kaempfen S, Trachsel D, Patole SK. Physical activity programs for promoting bone mineralization and growth in preterm infants. Cochrane Database Syst Rev. 2014:CD005387. https://doi.org/10.1002/14651858.
Eliakim A, Litmanovitz I, Nemet D. The role of exercise in prevention and treatment of osteopenia of prematurity: an update. Pediatr Exerc Sci. 2017;29:450–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kavurt, S., Demirel, N., Yücel, H. et al. Evaluation of radiologic evidence of metabolic bone disease in very low birth weight infants at fourth week of life. J Perinatol 41, 2668–2673 (2021). https://doi.org/10.1038/s41372-021-01065-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-021-01065-y