Abstract
Objective
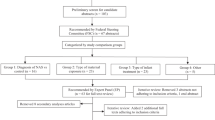
To assess if treating neonatal abstinence syndrome (NAS) with sublingual buprenorphine (SLB) would decrease the mean duration of therapy (DOT) and length of birth hospital stay (LOS).
Study design
Conducted at a tertiary hospital with >6000 annual deliveries and a 2% incidence of NAS, a quality improvement study using plan-do-study-act (PDSA) cycles were utilized. Outcomes were measured using statistical process control (SPC) charts.
Results
All NAS patients were treated with SLB, no adverse reactions were reported and the need for an adjunctive agent was static. SPC charts demonstrated decreased variability and special cause variation indicating a reduction in both DOT (from 14.5 to 8.5 days) and LOS (from 18.5 to 13 days).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kocherlakota P. Neonatal abstinence syndrome. Pediatrics. 2014;134:e547–6. https://doi.org/10.1542/peds.2013-3524
Winkelman T, Villapiano N, Copper W, Davis M, Patrick S. Incidence and costs of neonatal abstinence syndrome among infants with Medicaid: 2004–2014. Pediatrics. 2018;141:e20173520 https://doi.org/10.1542/peds.2017-3520.
Jones HE, Kaltenbach K, Heil SH, Stine SM, Coyle MG, Arria AM. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N Engl J Med. 2010;363:2320–31. https://doi.org/10.1056/NEJMoa1005359.
Coyle MG, Salisbury AL, Lester BM, Jones HE, Lin H, Graf-Rohrmeister K, et al. Neonatal neurobehavioral effects following buprenorphine vs methadone exposure. Addiction. 2012;107:63–73. https://doi.org/10.1111/j.1360-0443.2012.04040.x
Bier JB, Finger AS, Bier BA, Johnson TA, Coyle MG. Growth and developmental outcome of infants with in-utero exposure to methadone vs buprenorphine. J Perinatol. 2015;35:656–9. https://doi.org/10.1038/jp.2015.22
Dooley J, Gerber-Finn L, Antone I, Guilfoyle J, Blakelock B, Balfour-Boehm J. Buprenorphine-naloxone use in pregnancy for treatment of opioid dependence: retrospective cohort study of 30 patients. Can Fam Physician. 2016;62(Apr):e194–200.
Anagnostis EA, Sadaka RE, Sailor LA, Moody DE, Dysart KC, Kraft WK. Formulation of buprenorphine for sublingual use in neonates. J Pediatr Pharm Ther. 2011;16(Oct):281–4. https://doi.org/10.5863/1551-6776-16.4.281
Kraft WK, Dysart K, Greenspan JS, Gibson E, Kaltenbach K, Ehrlich ME. Revised dose schema of sublingual buprenorphine in the treatment of the neonatal opioid abstinence syndrome. Addiction. 2011;106:574–80. https://doi.org/10.1111/j.1360-0443.2010.03170.x
Ng CM, Dombrowsky E, Lin H, Erlich ME, Moody DE, Barrett JS, et al. Population pharmacokinetic model of sublingual buprenorphine in neonatal abstinence syndrome. Pharmacotherapy. 2015;35(Jul):670–80. https://doi.org/10.1002/phar.1610. Epub 2015 Jul 14
Moore JN, Gastonguay MR, Ng CM, Adeniyi-Jones SC, Moody DE, Fang WB, et al. The pharmacokinetics and pharmacodynamics of buprenorphine in neonatal abstinence syndrome. Clin Pharm Ther. 2018;103(Jun):1029–37. https://doi.org/10.1002/cpt.1064
Kraft WK, Gibson E, Dysart K, Damle VS, Larusso JL, Greenspan JS, et al. Sublingual buprenorphinfor treatment of neonatal abstinence syndrome: a randomized trial. Pediatrics. 2008;122:e601–e607. https://doi.org/10.1542/peds.2008-0571
Kraft WK, Adeniyi-Jones SC, Chervoneva I, Greenspan JS, Abatemarco D, Kaltenbach K, et al. Buprenorphine for the treatment of the neonatal abstinence syndrome. N Engl J Med. 2017;376:2341–8. https://doi.org/10.1056/NEJMoa161483
Hall ES, Isemann BT, Wexelblatt SL, Meinzen-Derr J, Wiles JR, Harvey S, et al. A cohort comparison of buprenorphine versus methadone treatment for neonatal abstinence syndrome. J Pediatr. 2016;170:e1 https://doi.org/10.1016/j.jpeds.2015.11.039
Hall ES, Rice WR, Folger AT, Wexelblatt SL. Comparison of neonatal abstinence syndrome treatment with sublingual buprenorphine versus conventional opioids. Am J Perinatol. 2018;35:405–12. https://doi.org/10.1055/s-0037-1608634
Porcelli P, Lambeth TM, Holmes A, Rojas M. Sustainability of improved practice guidelines and educational progress to reduce length of stay for treatment of narcotic abstinence syndrome. PAS 2016. Poster Presentation.
Shaughnessy EE, Shah A, Ambroggio L, Statile A. Quality improvement feature series article 1: introduction to quality improvement. J Pediatr Infect Dis Soc. 2018;7:6–10. https://doi.org/10.1093/jpids/pix061
Brady PW, Tchou MJ, Ambroggio L, Schondelmeyer AC, Shaughnessy EE. Quality improvement feature series article 2: displaying and analysing quality improvement data. J Pediatr Infect Dis Soc. 2018;7:100–3. https://doi.org/10.1093/jpids/pix077
Provost LP, Murray S. The health care data guide: learning from data for improvement. San Francisco: Jossey-Bass; 2011.
Benneyan JC, Lloyd RC, Plsek PE. Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care. 2003;12:458–64. https://doi.org/10.1136/qhc.12.6.458
MacMillan KDL, Rendon CP, Verma K, Riblet N, Washer DB, Volpe Holmes A. Association of rooming-in with outcomes for neonatal abstinence syndrome: a systematic review and meta-analysis. JAMA Pediatr. 2018;172:345–51. https://doi.org/10.1001/jamapediatrics.2017.5195
Marek E, Adeniyi-Jones SC, Roke L, DeCerbo TE, Cordell RL. Ethanol pharmacokinetics in neonates secondary to medication administration. Pediatrics. 2014;137:499A–499A.https://doi.org/10.1542/peds.137.Supplement_3.499A.
Oei JL, Melhuish E, Uebel H, Azzam N, Breen C, Burns L, et al. Neonatal abstinence syndrome and high school performance. Pediatrics. 2017;139:e20162651 https://doi.org/10.1542/peds.2016-2651
Behnke M, Smith VC. Committee on substance abuse; committee on fetus and newborn. Prenatal substance abus short- and long-term effects on the exposed fetus. Pediatrics. 2013;131:e1009–e1024. https://doi.org/10.1542/peds.2012-3931
Acknowledgements
Dr. Jennifer Check for her assistance with statistical analysis.
Author information
Authors and Affiliations
Contributions
SB helped conceptualize and design the study, played a key role in implementation, data collection, analyses and drafted the initial manuscript, and reviewed and revised the manuscript. TL played a key role in the QI design and analysis of the data, assisted in the implementation, and reviewed and revised the manuscript. AH helped make the product available through pharmacy, assisted in monitoring compliance in the use of SLB on the floor, and reviewed and revised the manuscript. MP conceptualized and helped design the study, assisted in the implementation, and critically reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bhandary, S., Lambeth, T., Holmes, A. et al. Using buprenorphine to treat neonatal abstinence syndrome: a quality improvement study. J Perinatol 41, 1480–1486 (2021). https://doi.org/10.1038/s41372-021-01035-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-021-01035-4