Abstract
Objective
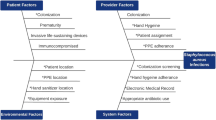
To evaluate the impact of active surveillance cultures (ASC) for Staphylococcus aureus (SA) and decolonization on the rate of infection in neonates in a neonatal intensive care unit (NICU).
Study design
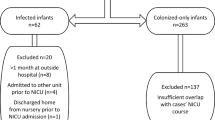
Using a quasi-experimental design with control groups, rates of SA infections before and after implementing weekly ASC and topical mupirocin decolonization in a level IV NICU were compared. Comparators were the rates of gram negative bloodstream infections (BSI) and of SA BSI at an affiliated NICU where the intervention was not implemented.
Result
There was a 77% (p < 0.010) reduction in rate of NICU-wide methicillin-susceptible SA (MSSA) BSI, but no significant change in rate of methicillin-resistant SA BSI, likely due to a prevalent mupirocin-resistant clone. Rates of gram negative BSI and SA BSI at an affiliated NICU did not change significantly.
Conclusion
Weekly ASC and decolonization were associated with a unit-wide reduction in MSSA infections in a NICU.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Hornik CP, Fort P, Clark RH, Watt K, Benjamin DK Jr, Smith PB, et al. Early and late onset sepsis in very-low-birth-weight infants from a large group of neonatal intensive care units. Early Hum Dev. 2012;88:S69–74.
Hocevar SN, Edwards JR, Horan TC, Morrell GC, Iwamoto M, Lessa FC. Device-associated infections among neonatal intensive care unit patients: incidence and associated pathogens reported to the National Healthcare Safely Network, 2006–2008. Infect Control Hosp Epidemiol. 2012;33:1200–6.
Greenberg RG, Kandelfer S, Do BT, Smith PD, Stoll BJ, Bell EF, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Late-onset sepsis in extremely premature infants: 2000–2011. Pediatr Infect Dis J. 2017;36:774–9.
Weiner-Lastinger LM, Abner S, Edwards JR, Kallen AJ, Karlsson M, Magill SS, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network, 2015-2017. Infect Control Hosp Epidemiol. 2020;41:19–30.
Shane AL, Hansen NI, Stoll BJ, Bell EF, Sanchez PJ, Shankaran S, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Methicillin-resistant and susceptible Staphylococcus aureus bacteremia and meningitis in preterm infants. Pediatrics. 2012;129:e914–22.
Al Shaik B, Yusuf K, Sauve R. Neurodevelopmental outcomes of very low birth weight infants with neonatal sepsis: systematic review and meta-analysis. J Perinatol. 2013;33:558–64.
Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, et al. National Institute of Child Health and Human Development Neonatal Research Network. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292:2357–65.
Ericson JE, Popoola VO, Smith PB, Benjamin DK, Fowler VG, Benjamin Jr DK, et al. Burden of invasive Staphylococcus aureus infections in hospitalized infants. JAMA Pediatr. 2015;169:1105–11.
Carey AJ, Duchon J, Della-Latta P, Saiman L. The epidemiology of methicillin-susceptible and methicillin-resistant Staphylococcus aureus in a neonatal intensive care unit, 2000-2007. Perinatol. 2010;30:135–9.
Graham PL, Morel AS, Zhou J, Wu F, Della-Latta P, Rubenstein D, et al. Epidemiology of methicillin-susceptible Staphylococcus aureus in the neonatal intensive care unit. Infect Control Hosp Epidemiol. 2002;23:677–82.
Popoola VO, Colantuoni E, Suwantarat N, Pierce R, Carroll KC, Aucott SW, et al. Active surveillance cultures and decolonization to reduce Staphylococcus aureus infections in the neonatal intensive care unit. Infect Control Hosp Epidemiol. 2016;37:381–7.
Huang YC, Chou YH, Su LH, Lien RI, Lin TY. Methicillin-resistant Staphylococcus aureus colonization and its association with infection among infants hospitalized in neonatal intensive care units. Pediatrics. 2006;118:469–74.
Milstone AM, Budd A, Shepard JW, Ross T, Aucott S, Carroll KC, et al. Role of decolonization in a comprehensive strategy to reduce methicillin-resistant Staphylococcus aureus infections in the neonatal intensive care unit: an observational cohort study. Infect Control Hosp Epidemiol. 2010;31:558–60.
Sakiki H, Nishioka M, Kanad K, Takahashi Y. An investigation of the risk factors of infection with methicillin-resistant Staphylococcus aureus among patients in a neonatal intensive care unit. Am J Infect Control. 2009;37:580–6.
Huang YC, Lien RI, Su LH, Chou YH, Lin TY. Successful control of methicillin-resistant Staphylococcus aureus in endemic neonatal intensive care units—a 7-year campaign. PloS ONE. 2011;6:e23001.
Delaney HM, Wang E, Melish M. Comprehensive strategy including prophylactic mupirocin to reduce Staphylococcus aureus colonization and infection in high-risk neonates. J Perinatol. 2013;33:313–8.
Wisgrill L, Zizka J, Unterasinger L, Rittenschober-Bӧhm J, Waldhӧr T, Makristathis A, et al. Active surveillance cultures and targeted decolonization are associated with reduced methicillin-susceptible Staphylococcus aureus infections in VLBW Infants. Neonatology. 2017;112:267–73.
Voskertchian A, Akinboyo IC, Colantuoni E, Johnson J, Milstone AM. Association of an active surveillance and decolonization program on incidence of clinical cultures growing Staphylococcus aureus in the neonatal intensive care unit. Infect Control Hosp Epidemiol. 2018;39:882–3.
Kotloff KL, Shirley DT, Creech CB, Frey SE, Harrison CJ, Staat M, et al. Mupirocin for Staphylococcus aureus decolonization of infants in neonatal intensive care units. Pediatrics. 2019;143:e20181565.
Akinboyo IC, Voskertchian A, Gorfu G, Betz JF, Ross TL, Carooll KC, et al. Epidemiology and risk factors for recurrent Staphylococcus aureus colonization following active surveillance and decolonization in the NICU. Infect Control Hosp Epidemiol. 2018;39:1334–9.
Kleinbaum DG, Kupper LL, Morgenstern H. Epidemiologic research: principles and quantitative methods. Belmont, California: Lifetime Learning Publications; 1982.
Rosenthal A, White D, Churilla S, Brodie S, Katz KC. Optimal surveillance culture sites for detection of methicillin-resistant Staphylococcus aureus in newborns. J Clin Microbiol. 2006;44:4234–6.
Wisgrill L, Berger A, Waldhӧr T, Makristathis A, Assadian O, Rittenschober-Bӧhm J. Combination of nasal and expanded skin swabs enhances the detection rate of Staphylococcus aureus colonization in premature infants. Pediatr Infect Dis J. 2019;38:422–32.
Gibbs K, Holzman IR. Endotracheal tube: friend or foe? bacteria, the endotracheal tube, and the impact of colonization and infection. Semin Perinatol. 2012;36:454–61.
Suwantarat N, Carroll KC, Tekle T, Ross T, Popoola VO, Milstone AM, et al. Low prevalence of mupirocin resistance among hospital-acquired methicillin-resistant Staphylococcus aureus isolates in a neonatal intensive care unit with an active surveillance cultures and decolonization program. Infect Control Hosp Epidemiol. 2015;36:232–4.
Pierce R, Bryant K, Elward A, Lessler J, Milstone AM. Bacterial infections in neonates following mupirocin-based MRSA decolonization: a multicentre cohort study. Infect Control Hosp Epidemiol. 2017;38:930–6.
Calfee DP, Salgado CD, Milstone AM, Harris AD, Kuhar DT, Moody J, et al. Society for healthcare epidemiology of America. Strategies to prevent methicillin-resistant Staphylococcus aureus transmission and infection in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35:772–96.
Milstone AM, Voskertchian A, Koontz DW, Khamash DF, Ross T, Aucott SW, et al. Effect of treating parents colonized with Staphylococcus aureus on transmission to neonates in the intensive care unit: a randomized clinical trial. JAMA. 2020;323:319–28.
Acknowledgements
We would like to thank the staff of the microbiology lab of the Long Island Jewish Medical Center, Northwell Health, New Hyde Park, New York for their support and cooperation.
Author information
Authors and Affiliations
Contributions
AB conceptualized the study, designed the data collection instruments, collected data, drafted the initial paper and reviewed and revised the paper. LGR conceptualized and designed the study, coordinated and supervised data collection, and critically reviewed the paper for important intellectual concept. JB conceptualized and designed the study and reviewed and revised the paper. NK supervised the data collection instruments, carried out the statistical analyses and reviewed and revised the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Balamohan, A., Beachy, J., Kohn, N. et al. The effect of routine surveillance and decolonization on the rate of Staphylococcus aureus infections in a level IV neonatal intensive care unit. J Perinatol 40, 1644–1651 (2020). https://doi.org/10.1038/s41372-020-0755-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-020-0755-5