Abstract
Objective
To assess the association of cerebral oxygen saturation (CrSO2) collected by near infrared spectroscopy (NIRS) during therapeutic hypothermia (TH) and rewarming with evidence of brain injury on post-rewarming MRI.
Study design
This retrospective cohort study included 49 infants, who received TH for mild to severe neonatal encephalopathy. Of those, 26 presented with brain injury assessed by a novel MRI grading system, whereas 23 had normal MRI scans.
Results
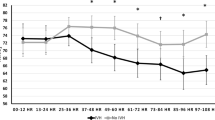
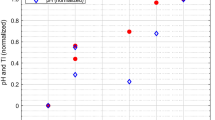
CrSO2 increased significantly from the first to the second day of TH in infants with brain injury, whereas it remained stable in patients with normal MRI. Increasing mean CrSO2 values during rewarming was associated with brain injury (aOR 1.14; 95% CI 1.00–1.28), specifically with gray matter (GM) injury (aOR 1.23; 95% CI 1.02–1.49). The area under the ROC curve showed an excellent discrimination for GM involvement.
Conclusion
Clinically applied NIRS during TH and rewarming can assist in identifying the risk for brain injury.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum Dev. 2010;86:329–38.
Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2013;1:CD003311.
Natarajan G, Pappas A, Shankaran S. Outcomes in childhood following therapeutic hypothermia for neonatal hypoxic-ischemic encephalopathy (HIE). Semin Perinatol. 2016;40:549–55.
El-Dib M, Soul JS. Monitoring and management of brain hemodynamics and oxygenation. Handb Clin Neurol. 2019;162:295–314.
Wolf M, Greisen G. Advances in near-infrared spectroscopy to study the brain of the preterm and term neonate. Clin Perinatol. 2009;36:807–34.
Pryds O, Greisen G, Lou H, Friis-Hansen B. Vasoparalysis associated with brain damage in asphyxiated term infants. J Pediatr. 1990;117:119–25.
Toet MC, Lemmers PM, van Schelven LJ, van Bel F. Cerebral oxygenation and electrical activity after birth asphyxia: their relation to outcome. Pediatrics. 2006;117:333–9.
Yenari MA, Han HS. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat Rev Neurosci. 2012;13:267–78.
Ancora G, Maranella E, Grandi S, Sbravati F, Coccolini E, Savini S, et al. Early predictors of short term neurodevelopmental outcome in asphyxiated cooled infants. A combined brain amplitude integrated electroencephalography and near infrared spectroscopy study. Brain Dev. 2013;35:26–31.
Lemmers PM, Zwanenburg RJ, Benders MJ, de Vries LS, Groenendaal F, van Bel F, et al. Cerebral oxygenation and brain activity after perinatal asphyxia: does hypothermia change their prognostic value? Pediatr Res. 2013;74:180–5.
Shellhaas RA, Thelen BJ, Bapuraj JR, Burns JW, Swenson AW, Christensen MK, et al. Limited short-term prognostic utility of cerebral NIRS during neonatal therapeutic hypothermia. Neurology. 2013;81:249–55.
Goeral K, Urlesberger B, Giordano V, Kasprian G, Wagner M, Schmidt L, et al. Prediction of outcome in neonates with hypoxic-ischemic encephalopathy II: role of amplitude-integrated electroencephalography and cerebral oxygen saturation measured by near-infrared spectroscopy. Neonatology. 2017;112:193–202.
Hunter CL, Oei JL, Suzuki K, Lui K, Schindler T. Patterns of use of near-infrared spectroscopy in neonatal intensive care units: international usage survey. Acta Paediatr. 2018;107:1198–204.
Weeke LC, Groenendaal F, Mudigonda K, Blennow M, Lequin MH, Meiners LC, et al. A novel magnetic resonance imaging score predicts neurodevelopmental outcome after perinatal asphyxia and therapeutic hypothermia. J Pediatrics. 2018;192:33–40 e2.
Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–84.
Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol. 1976;33:696–705.
Hellstrom-Westas L, Rosen I, Svenningsen NW. Predictive value of early continuous amplitude integrated EEG recordings on outcome after severe birth asphyxia in full term infants. Arch Dis Child Fetal Neonatal Ed. 1995;72:F34–8.
Meek JH, Elwell CE, McCormick DC, Edwards AD, Townsend JP, Stewart AL, et al. Abnormal cerebral haemodynamics in perinatally asphyxiated neonates related to outcome. Arch Dis Child Fetal Neonatal Ed. 1999;81:F110–5.
Gunn AJ, Thoresen M. Hypothermic neuroprotection. NeuroRx. 2006;3:154–69.
Cady EB, Amess P, Penrice J, Wylezinska M, Sams V, Wyatt JS. Early cerebral-metabolite quantification in perinatal hypoxic-ischaemic encephalopathy by proton and phosphorus magnetic resonance spectroscopy. Magn Reson Imaging. 1997;15:605–11.
Chiron C, Raynaud C, Maziere B, Zilbovicius M, Laflamme L, Masure MC, et al. Changes in regional cerebral blood flow during brain maturation in children and adolescents. J Nucl Med. 1992;33:696–703.
Chugani HT. A critical period of brain development: studies of cerebral glucose utilization with PET. Prev Med. 1998;27:184–8.
Bouyssi-Kobar M, Murnick J, Brossard-Racine M, Chang T, Mahdi E, Jacobs M, et al. Altered cerebral perfusion in infants born preterm compared with infants born full term. J Pediatrics. 2018;193:54–61 e2.
Massaro AN, Bouyssi-Kobar M, Chang T, Vezina LG, du Plessis AJ, Limperopoulos C. Brain perfusion in encephalopathic newborns after therapeutic hypothermia. AJNR Am J Neuroradiol. 2013;34:1649–55.
Gebauer CM, Knuepfer M, Robel-Tillig E, Pulzer F, Vogtmann C. Hemodynamics among neonates with hypoxic-ischemic encephalopathy during whole-body hypothermia and passive rewarming. Pediatrics. 2006;117:843–50.
Wu TW, Tamrazi B, Soleymani S, Seri I, Noori S.Hemodynamic changes during rewarming phase of whole-body hypothermia therapy in neonates with hypoxic-ischemic encephalopathy. J Pediatrics. 2018;197:68–74 e2.
Bacher A. Effects of body temperature on blood gases. Intensive Care Med. 2005;31:24–7.
Chalak LF, Tarumi T, Zhang R. The “neurovascular unit approach” to evaluate mechanisms of dysfunctional autoregulation in asphyxiated newborns in the era of hypothermia therapy. Early Hum Dev. 2014;90:687–94.
Peng S, Boudes E, Tan X, Saint-Martin C, Shevell M, Wintermark P. Does near-infrared spectroscopy identify asphyxiated newborns at risk of developing brain injury during hypothermia treatment? Am J Perinatol. 2015;32:555–64.
Alderliesten T, Dix L, Baerts W, Caicedo A, van Huffel S, Naulaers G, et al. Reference values of regional cerebral oxygen saturation during the first 3 days of life in preterm neonates. Pediatr Res. 2016;79:55–64.
Lin PY, Roche-Labarbe N, Dehaes M, Carp S, Fenoglio A, Barbieri B, et al. Non-invasive optical measurement of cerebral metabolism and hemodynamics in infants. J Vis Exp. 2013;73:e4379.
Dehaes M, Aggarwal A, Lin PY, Rosa Fortuno C, Fenoglio A, Roche-Labarbe N, et al. Cerebral oxygen metabolism in neonatal hypoxic ischemic encephalopathy during and after therapeutic hypothermia. J Cereb Blood Flow Metab. 2014;34:87–94.
Bale G, Mitra S, Meek J, Robertson N, Tachtsidis I. A new broadband near-infrared spectroscopy system for in-vivo measurements of cerebral cytochrome-c-oxidase changes in neonatal brain injury. Biomed Opt Express. 2014;5:3450–66.
Bale G, Mitra S, de Roever I, Sokolska M, Price D, Bainbridge A, et al. Oxygen dependency of mitochondrial metabolism indicates outcome of newborn brain injury. J Cereb Blood Flow Metab. 2019;39:2035–47.
El-Dib M, Inder TE, Chalak LF, Massaro AN, Thoresen M, Gunn AJ. Should therapeutic hypothermia be offered to babies with mild neonatal encephalopathy in the first 6 h after birth? Pediatr Res. 2019;85:442–8.
Thayyil S, Chandrasekaran M, Taylor A, Bainbridge A, Cady EB, Chong WK, et al. Cerebral magnetic resonance biomarkers in neonatal encephalopathy: a meta-analysis. Pediatrics. 2010;125:e382–95.
Author information
Authors and Affiliations
Contributions
ES had substantial contributions to conception and design, data analysis, and interpretation of data. She also drafted the article and had final approval of the version to be published. JS helped to collect and analyze the data and to write the manuscript draft. EY and TI analyzed MRI imaging studies, supervised the interpretation of data and revised the manuscript. JJV had substantial contributions to conception and interpretation of data, and revised the manuscript for important intellectual content. ME conceptualized and designed the study, supervised all aspects and critically reviewed the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
ES, TI, and ME-D received an External Research Grant from Medtronic, Inc to support a clinical study not related to the work presented in this manuscript. The authors have no financial relationships relevant to this article to disclose.
Ethics approval and consent to participate
This study was approved by the Partners Human Research Committee Institutional Review Board and consent was waived (IRB protocol# 2014P002430).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Szakmar, E., Smith, J., Yang, E. et al. Association between cerebral oxygen saturation and brain injury in neonates receiving therapeutic hypothermia for neonatal encephalopathy. J Perinatol 41, 269–277 (2021). https://doi.org/10.1038/s41372-020-00910-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-020-00910-w
This article is cited by
-
The predictive value of MRI scores for neurodevelopmental outcome in infants with neonatal encephalopathy
Pediatric Research (2024)
-
Neuromonitoring in neonatal critical care part I: neonatal encephalopathy and neonates with possible seizures
Pediatric Research (2023)
-
Comparative evaluation of approach to cardiovascular care in neonatal encephalopathy undergoing therapeutic hypothermia
Journal of Perinatology (2022)