Abstract
Objective
To systematically review the studies exploring the association between bevacizumab and neurodevelopmental outcomes.
Methods
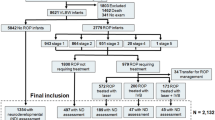
Embase, Medline, CINAHL, and Cochrane Library databases were searched for studies examining neurodevelopmental outcomes of preterm infants treated with bevacizumab compared to laser ablation or cryotherapy for severe retinopathy of prematurity (ROP).
Results
Thirteen studies (clinical trial = 1; cohort studies = 12) were included. Random-effects model meta-analysis showed significant increased odds of cognitive impairment associated with bevacizumab treatment on both unadjusted (unadjusted odds ratio (OR) 1.61; 95% confidence interval (CI) 1.12, 2.30) and adjusted analyses (adjusted OR 1.90; 95% CI 1.22, 2.97). Infants treated with bevacizumab for severe ROP had significantly lower Bayley-III cognitive (mean difference (MD) −1.66; 95% CI −3.21, −0.12), and language composite scores (MD −5.50; 95% CI −8.24, −2.76) compared to infants treated with laser ablation or cryotherapy.
Conclusion
Bevacizumab treatment for severe ROP is associated with increased risk of cognitive impairment and lower cognitive and language scores in preterm infants.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ann Hellström Lois EH Smith, Olaf Dammann. Retinopathy of prematurity. The Lancet. 2013;382:1445–57.
Msall ME, Phelps DL, DiGaudio KM, Dobson V, Tung B, McClead RE, et al. Severity of neonatal retinopathy of prematurity is predictive of neurodevelopmental functional outcome at age 5.5 years. Pediatrics. 2000;106:998–1005.
Schmidt B, Davis PG, Asztalos EV, Solimano A, Roberts RS. Association between severe retinopathy of prematurity and nonvisual disabilities at age 5 years. JAMA. 2014;311:523–5.
Glass TJA, Chau V, Gardiner J, Fonng J, Vinall J, Zwicker JG, et al. Severe retinopathy of prematurity predicts delayed white matter maturation and poorer neurodevelopment. Arch Dis Child Fetal Neonatal Ed. 2017;102:F532–7.
Msall ME. The retina as a window to the brain in vulnerable neonates. Pediatrics. 2006;117:2287–9.
Mintz-Hittner HA. Treatment of retinopathy of prematurity with vascular endothelial growth factor inhibitors. Early Hum Dev. 2012;88:937–41.
Mintz-Hittner HA, Kennedy KA, Chuang AZ, BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N. Engl J Med. 2011;364:603–15.
Cernichiaro-Espinosa LA, OlguinManriquez FJ, Henaine-Berra A, GarciaAguirre G, Quiroz-Mercado H, MartinezCastellanos MA. New insights in diagnosis and treatment for retinopathy of prematurity. Int Ophthalmol. 2016;36:751–60.
Good WV. Early treatment for retinopathy of prematurity cooperative group. final results of the early treatment for retinopathy of prematurity (ETROP) randomized trial. Trans Am Ophthalmol Soc. 2004;102:233–48.
Sankar MJ, Sankar J, Chandra P. Anti-vascular endothelial growth factor (VEGF) drugs for treatment of retinopathy of prematurity. Cochrane Database Syst Rev. 2018;1:CD009734.
Micieli JA, Surkont M, Smith AF. A systematic analysis of the off-label use of bevacizumab for severe retinopathy of prematurity. Am J Ophthalmol. 2009;148:536.
Hu J, Blair MP, Shapiro MJ, Lichtenstein SJ, Galasso JM, Kapur R. Reactivation of retinopathy of prematurity after bevacizumab injection. Arch Ophthalmol. 2012;130:1000.
Hwang CK, Hubbard GB, Hutchinson AK, Lambert SR. Outcomes after intravitreal bevacizumab versus laser photocoagulation for retinopathy of prematurity: a 5-year retrospective analysis. Ophthalmology. 2015;122:1008–15. Epub 2015 Feb 14.
Nicoară SD, Nascutzy C, Cristian C, Irimescu I, Ștefănuț AC, Zaharie G, et al. Outcomes and prognostic factors of intravitreal bevacizumab monotherapy in zone I Stage 3+ and aggressive posterior retinopathy of prematurity. J Ophthalmol. 2015;2015:102582. Epub 2015 9 27.
Chen SN, Lian I, Hwang YC, Chen YH, Chang YC, Lee KH, et al. Intravitreal anti-vascular endothelial growth factor treatment for retinopathy of prematurity: comparison between Ranibizumab and Bevacizumab. Retina. 2015;35:667–74.
Fleck BW. Management of retinopathy of prematurity. Arch Dis Child Fetal Neonatal Ed. 2013;98:F454. Epub 2013 Jun 27
Reynolds JD. Bevacizumab for retinopathy of prematurity. N. Engl J Med. 2011;364:677.
Wu WC, Lien R, Liao PJ, Wang NK, Chen YP, Chao AN, et al. Serum levels of vascular endothelial growth factor and related factors after intravitreous bevacizumab injection for retinopathy of prematurity. JAMA Ophthalmol. 2015;133:391–7.
Lepore D, Quinn GE, Molle F, Baldascino A, Orazi L, Sammartino M, et al. Intravitreal bevacizumab versus laser treatment in type 1 retinopathy of prematurity: report on fluorescein angiographic findings. Ophthalmology. 2014;121:2212–2219 6.
Martinez-Castellanos MA, Schwartz S, Hernandez-Rojas ML, Kon-Jara VA, Garcia-Aguirre G, Guer- rero-Naranjo JL, et al. Long-term effect of antiangiogenic therapy for retinopathy of prematurity up to 5 years of follow-up. Retina. 2013;33:329–38.
Jalali S, Balakrishnan D, Zeynalova Z, Padhi TR, Rani PK. Serious adverse events and visual outcomes of rescue therapy using adjunct bevacizumab to laser and surgery for retinopathy of prematurity. The Indian Twin Cities Retinopathy of Prematurity Screening database report number 5. Arch Dis Child Fetal Neonatal Ed. 2013;98:F327–33.
Käll A. Is Avastin the right choice of treatment for retinopathy of prematurity? Acta Paediatr. 2012;101:796–8.
Darlow BA, Ells AL, Gilbert CE, Gole GA, Quinn GE. Are we there yet? Bevacizumab therapy for retinopathy of prematurity. Arch Dis Child Fetal Neonatal Ed. 2013;98:F170–4.
Sato T, Wada K, Arahori H, Kuno M, Imoto K, Iwashashi-Shima C, et al. Serum concentrations of bevacizumab (avastin) and vascular endothelial growth factor in infants with retinopathy of prematurity. Am J Ophthalmol. 2012;153:327–33.
Kennedy KA, Mintz-Hittner HA. Medical and developmental outcomes of bevacizumab versus laser for retinopathy of prematurity. J AAPOS. 2018;22:61–5.e1.
Raghuram K, Isaac M, Yang J, AlAli A, Mireskandari K, Ly LG, et al. Neurodevelopmental outcomes in infants treated with intravitreal bevacizumab versus laser. J Perinatol. 2019;39:1300–8.
Rodriguez SH, Peyton C, Lewis K, Andrews B, Greenwald MJ, Schreiber MD, et al. Neurodevelopmental outcomes comparing bevacizumab to laser for type 1 ROP. Ophthalmic Surg Lasers Imaging. Retina. 2019;50:337–43.
Natarajan G, Shankaran S, Nolen TL, Sridhar A, Kennedy KA, Hintz SR, et al. Neurodevelopmental outcomes of preterm infants with retinopathy of prematurity by treatment. Pediatrics. 2019;144:e20183537.
Morin J, Luu TM, Superstein R, Ospina LH, Lefebvre F, Simard M-N, et al. Neurodevelopmental outcomes following bevacizumab injections for retinopathy of prematurity. Pediatrics. 2016;137:4.
Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. Chichester, West Sussex, UK: Wiley; 2011.
Hardy RJ, Palmer EA, Dobson V, Summers CG, Phelps DL, Quinn GE. Cryotherapy for Retinopathy of Prematurity Cooperative Group et al. Risk analysis of prethreshold retinopathy of prematurity. Arch Ophthalmol. 2003;121:1697–170114662587.
Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214–23.
Bayley N. Bayley scales of infant development. 2nd ed. San Antonio, TX: Psychological Corporation; 1993.
Albers CA, Grieve AJ. Test Review: Bayley, N. (2006). Bayley scales of infant and toddler development– Third Edition. San Antonio, TX: Harcourt Assessment. Psychol Assess. 2007;25:180–90.
Cochrane. GRADE handbook for grading quality of evidence and strength of recommendations. In: Schünemann H, Brożek J, Guyatt G, Oxman A, editors. McMaster University, Canada: Cochrane; 2015.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135.
Arima M, Akiyama M, Fujiwara K, Mori Y, Inoue H, Seki E, et al. Neurodevelopmental outcomes following intravitreal bevacizumab injection in Japanese preterm infants with type 1 retinopathy of prematurity. PLoS One. 2020;15:e0230678.
Lien R, Yu M-H, Hsu K-H, Liao P-J, Chen Y-P, Lai C-C, et al. Neurodevelopmental outcomes in infants with retinopathy of prematurity and bevacizumab treatment. PLoS One. 2016;11:e0148019.
Zayek M, Parker K, Rydzewska M, Rifai A, Bhat R, Eyal F, et al. Bevacizumab for retinopathy in preterm infants: two-year developmental follow-up. J Investig Med. 2020;68:588.
Tiffany A, Chen BS, Ira H, Schachar MD, Darius M, Moshfeghi MD. Outcomes of intravitreal bevacizumab and diode laser photocoagulation for treatment-warranted retinopathy of prematurity. Ophthalmic Surg Lasers Imaging Retin. 2018;49:126–31.
Mantagos S, Wu C, Winter T. Mortality rate for premature infants treated for rop with intravitreal antivascular endothelial growth factor (VEGF) medication vs retinal ablative surgery. J AAPOS. 2017;21:e18.
Kong L, Dinh K, Schechet S, Coats D, Voigt R, Demny A, et al. Comparison of ocular and developmental outcomes in laser-and bevacizumab-treated infants with retinopathy of prematurity. Ophthalmol Res Int J. 2015;3:13–22.
Anand N, Blair MP, Greenwald MJ, Rodriguez SH. Refractive outcomes comparing primary laser to primary bevacizumab with delayed laser for type 1 ROP. J AAPOS. 2019;23:88E1–6.
Huddleston SM, Calderwood J, Hoehn ME. Comparing morbidity rates in retinopathy of prematurity treated with either intravitreal bevacizumab or conventional laser therapy. Investig Ophthalmol Vis Sci. 2014;55:2053.
Sato T, Wada K, Arahori H, Kuno N, Imotot K, Iwahashu-Shima C, et al. Serum concentrations of bevacizumab (Avastin) and vascular endothelial growth factor in infants with retinopathy of prematurity. Am J Ophthalmol. 2012;153:327–33.
Kong L, Bhatt AR, Demny AB, Coats D, Li A, Rahman EZ, et al. Pharmacokinetics of bevacizumab and its effects on serum VEGF and IFG-1 in infants with retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2015;56:956–61.
Wu W, Shih C, Lien R, Wang N, Chen Y, Chao A, et al. Serum vascular endothelial growth factor after bevacizumab or ranibizumab treatment for retinopathy of prematurity. Retina. 2016;0:1–8.
Rosenstein JM, Krum JM, Ruhrberg C. VEGF in the nervous system. Organogenesis. 2010;6:107–14.
Eichmann A, Thomas JL. Molecular parallels between neural and vascular development. Cold Spring Harb Perspect Med. 2013;3:1–16.
Stahl A, Lepore D, Fielder A, Fleck B, Reynolds J, Chiang M, et al. Ranibizumab versus laser therapy for the treatment of very low birthweight infants with retinopathy of prematurity (RAINBOW): an open-label randomised controlled trial. Lancet. 2019;394:1551–9.
Funding
This research was funded by the Deanship of Scientific Research at Princess Nourah Bint Abdulrahman University through the Fast track Research Funding Program. The funder had no role in conceiving the study design, collection, analysis and interpretation of the data, and decision to publish the study.
Author information
Authors and Affiliations
Contributions
MK and WP had full access to the data and take responsibility for the data integrity including accuracy of the data analysis. Concept/design: AR, MK, WP, AK, AKP. Acquisition, analysis, and interpretation of data: AR, MK, WP, AK, and AKP. Initial drafting of the manuscript: AR and MK. Critical revision for important intellectual content: AR. Statistical analysis: AR, MK, and WP. Administrative, technical, or material support: AR. Study supervision: AR.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Kaushal, M., Razak, A., Patel, W. et al. Neurodevelopmental outcomes following bevacizumab treatment for retinopathy of prematurity: a systematic review and meta-analysis. J Perinatol 41, 1225–1235 (2021). https://doi.org/10.1038/s41372-020-00884-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-020-00884-9
This article is cited by
-
Current Management of Retinopathy of Prematurity
Current Treatment Options in Pediatrics (2022)
-
The UK practice of Anti-VEGF therapy for treatment of retinopathy of prematurity
Eye (2021)
-
Reply to letter to editor: Neurodevelopmental outcomes following bevacizumab treatment for retinopathy of prematurity: a systematic review and meta-analysis
Journal of Perinatology (2021)